ABSTRACT
- Korean Red ginseng has emerged as a potent candidate in the fight against various viral infections, demonstrating significant efficacy both in vitro and in vivo, particularly against influenza A viruses. Despite substantial evidence of its antiviral properties, the detailed molecular mechanisms through which it reduces viral lethality remain insufficiently understood. Our investigations have highlighted the superior effectiveness of Korean Red ginseng against influenza viruses, outperforming its effects on numerous other viral strains. We aim to uncover the specific mechanisms by which Korean Red ginseng exerts its antiviral effects, focusing on influenza A viruses. Our prior studies have identified the role of Z-DNA-binding protein 1 (ZBP1), a signaling complex involved in inducing programmed cell death in response to influenza virus infection. Given the critical role of ZBP1 as a sensor for viral nucleic acid, we hypothesize that Korean Red ginseng may modulate the ZBP1-derived cell death pathway. This interaction is anticipated to enhance cell death while concurrently suppressing viral protein expression, offering novel insights into the antiviral mechanism of Korean Red ginseng against influenza A viruses.
-
Keywords: cell death, host defense, influenza A virus, Red ginseng, ZBP1
Introduction
Korean Red ginseng is a thermally processed form of ginseng produced by repeatedly steaming and naturally drying fresh ginseng (Yun, 2001). This method induces alterations in chemical constituents, such as ginsenosides Rg2, Rg3, Rh1, and Rh2, resulting in Korean Red ginseng exhibiting enhanced pharmacological efficacy and stability relative to fresh ginseng (Kim et al., 2000). Numerous pharmacological benefits of ginseng and its components have been documented, including, anti-cancer (Qian et al., 2014; Sun et al., 2011; Yue et al., 2006; Yun, 2003), anti-allergic (Jung et al., 2013; Kim & Yang, 2011; Park et al., 2004; Sumiyoshi et al., 2010), anti-inflammatory (Ahn et al., 2006; Lee & Cho, 2011; Park et al., 2004, 2009; Zhao et al., 2024), anti-fatigue (Bao et al., 2016; Choi et al., 2011; Tan et al., 2013; Wang et al., 2010), immunomodulatory effects (Kenarova et al., 1990; Kim et al., 1990; Nakaya et al., 2004; Sung et al., 2005) and tumor cell growth inhibition (Ota et al., 1987, 1996; Tode et al., 1993), and cell death (Li et al., 2011; Park et al., 2012; Popovich & Kitts, 2002; Wakabayashi et al., 1998). Furthermore, the antiviral properties of ginseng have been substantiated, with Korean Red ginseng extracts and ginsenosides demonstrating antiviral activities against influenza virus (Lee et al., 2014a; Yoo et al., 2012a, 2012b), rhinovirus (Kim et al., 2024; Song et al., 2014), human immunodeficiency virus (Cho & Kim, 2017; Sung et al., 2005, 2009).
In particular, the antiviral properties of Korean Red ginseng play a crucial role in supporting the innate immune system. Innate immunity is the first line of defense activated when pathogens, such as viruses, invade the host. The host continuously maintains defensive and offensive mechanisms against pathogens of varying sizes and lethality. The innate immune process can be broadly divided into three stages with pathogen recognition, activation of signaling pathways, and establishment of an antiviral state. Upon pathogen recognition, signaling pathways are activated, leading to the induction of an antiviral state. Pathogen recognition is mediated by pattern recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Prominent PRRs include Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptor family proteins (NLRs), absent in melanoma 2 (AIM2), and Z-DNA binding protein 1 (ZBP1). This process involves the recruitment of immune cells such as macrophages and neutrophils, as well as the activation of programmed cell death (PCD) pathways to eliminate infected cells and inhibit viral spread (Ashida et al., 2011; Fink & Cookson, 2005; Lamkanfi & Dixit, 2010; Lee et al., 2018, 2019).
ZBP1, also referred to as DAI or DLM-1, is a nucleic acid sensor characterized by the presence of two Zα domains that bind to Z-DNA and Z-RNA (Ha et al., 2008; Maelfait et al., 2017; Placido et al., 2007; Schwartz et al., 2001; Thapa et al., 2016). ZBP1 plays a crucial role in mediating host defense against a variety of viruses through the recognition of viral nucleic acids. Recent research has identified ZBP1 as a central regulator of PANoptosis (Banoth et al., 2020; Karki et al., 2021b; Lee et al., 2021c; Oh & Lee, 2023), a unique form of programmed cell death essential for the defense against viral infections, including SARS-CoV-2 (Karki et al., 2021a, 2022; Zheng et al., 2020) and Influenza A virus (IAV) (Kesavardhana et al., 2020; Kuriakose et al., 2016; Thapa et al., 2016).
Korean Red ginseng has demonstrated significant efficacy against various viral infections (Lee et al., 2013, 2014b; Song et al., 2014), particularly influenza viruses, in both in vitro and in vivo studies. Despite the substantial evidence supporting its antiviral properties, the detailed molecular mechanisms underlying its reduction of viral lethality remain insufficiently elucidated. Here, we discovered that Korean Red ginseng modulates the ZBP1-derived cell death pathway, a mechanism hypothesized to enhance programmed cell death while simultaneously suppressing viral protein expression.
In our study, wild-type (WT) bone marrow-derived macrophages (BMDMs) infected with influenza A virus and treated with Korean Red ginseng exhibited increased cell death and reduced viral protein expression compared to untreated controls. This suggests that Korean Red ginseng enhances the ZBP1-derived cell death pathway. In contrast, Zbp1–/– BMDMs infected with the virus showed significantly reduced cell death and increased viral protein expression, indicating the critical role of ZBP1 in mediating this response.
To further investigate these findings, WT and Zbp1–/– mice were administered Korean Red ginseng for 7 days prior to being challenged with influenza A virus for 14 days. WT mice treated with Korean Red ginseng demonstrated improved survival rates compared to those infected with the virus alone. However, Zbp1–/– mice showed no significant differences in survival across all groups, reinforcing the role of ZBP1 in the antiviral effects of Korean Red ginseng. These results confirm that Korean Red ginseng enhances the ZBP1-derived cell death pathway, providing novel insights into its antiviral mechanism against influenza viruses.
Materials and Methods
Mice
C57BL/6J (wild type [WT]) (Hyochang Science) and Zbp1–/– (Cyagen) mice were purchased from the indicated sources. The mice were group-housed (up to five mice per cage), bred under standard pathogen-free housing conditions in the animal facility of Ulsan National Institute of Science and Technology (UNIST) under a 12 h light/dark cycle (lights on from 7 A.M. to 7 P.M.), and fed standard chow. Both male and female mice were used in this study. Age- and sex-matched 6- to 8-week-old mice were used for the in vivo studies, and 6- to 12-week-old mice were used for the in vitro studies. Cohoused animals were used for in vivo analyses. All the experimental procedures were conducted in accordance with protocols that were approved by the Institutional Animal Care and Utilization Committee of UNIST.
Cell culture
Primary bone marrow-derived macrophages (BMDMs) were cultivated for six days in IMDM (Thermo Fisher Scientific, 12440061) supplemented with 5% FBS (Thermo Fisher Scientific, 16000044), 30% L929-conditioned medium, 1% nonessential amino acids (Thermo Fisher Scientific, 11140-050), and 1% penicillin and streptomycin (Thermo Fisher Scientific, 15070-063). BMDMs were seeded in 12-well plates at a density of 1 million cells/well and incubated overnight before use. L929 (ATCC, CCL-1) were purchased and grown in IMDM supplemented with 10% FBS (Thermo Fisher Scientific, 16000044), 1% nonessential amino acids, and 1% penicillin and streptomycin. A549 (gift from Dr. Atsushi Kawaguchi [University of Tsukuba]) were grown in DMEM (Thermo Fisher Scientific, 11995081) supplemented with 5% FBS and 1% penicillin and streptomycin.
Virus culture
Influenza A virus A/Puerto Rico/8/34 (H1N1, PR8), A/Switzerland/9715293/2013 (H3N2, Switzerland/2013), and A/WSN/1933 (H1N1, WSN) was kindly gifted by Dr. Man-Seong Park (Korea University) and propagated in 11-day-old embryonated chicken eggs by allantoic inoculation. Human herpes simplex virus 1 (HF strain) (ATCC; VR-260) was purchased and propagated in Vero cells (Korean Cell Line Bank; 10081); the virus titer was measured using the plaque assay in Vero cells. Vaccinia virus (Western Reserve, WR) (ATCC; VR-1354) was purchased and propagated in Vero cells (Korean Cell Line Bank; 10081); the virus titer was measured using the plaque assay in Vero cells. Mumps rubulavirus (MuV/Iowa.US/2006) (ATCC; VR-1899) was purchased and propagated in Vero cells; the virus titer was measured using the plaque assay in Vero cells. HCoV-NL63 was purchased in KBPV (KBPV-VR-88) and propagated in Vero cells (Korean Cell Line Bank; 10081); the virus titer was measured using the plaque assay in Vero cells.
Cell infection and stimulation
For HSV-1 (30 h; MOI 10), VACV (30 h; MOI 10), HCoV-NL63 (30 h; MOI 0.3), and MuV (30 h; MOI 10) infection, cells were infected in DMEM high glucose (Gibco, 11995-065). For IAV (15 h or 30 h; MOI 10) infection, cells were infected in high-glucose DMEM (WELGENE, LM001-03). Korean Red ginseng extracts are provided by Korea Ginseng Corporation (KGC) are added to cells after 1 h of infection.
Real-time cell death analysis
An IncuCyte S3 imaging system (Sartorius) was purchased, and real-time cell death analysis was performed as previously using IncuCyte S3 imaging system (Essen Biosciences). BMDMs were seeded in 12-well plates (106 cells/well) and stimulated. After incubation, SYTOX Green (Thermo Fisher Scientific, S7020) was added according to the manufacturer’s protocol. Images were acquired every 1 h at 37°C in 5% CO2. The resulting images were analyzed using the software supplied with the IncuCyte imager, the number of SYTOX Green-positive BMDM nuclei (Sytox+ BMDM nuclei) and SYTOX Green-positive A549 nuclei (Sytox+ A549 nuclei) present in each image were counted.
Immunoblot analysis and quantification
Immunoblotting was performed as previously described (Karki et al., 2022; Lee et al., 2021b; Oh et al., 2023; Wang et al., 2021a, 2022a). Briefly, for caspase analysis, BMDMs were lysed along with their supernatant using 50 μl caspase lysis buffer (1×protease inhibitors, 1× phosphatase inhibitors, 10% NP-40, and 25 mM DTT), followed by the addition of 100 μl 4 × SDS loading buffer. For signalling protein analysis, BMDM supernatants were removed at the indicated time points, the cells were washed once with PBS, and the cells were then lysed with RIPA buffer. Proteins were separated by electrophoresis in 8–15% polyacrylamide gels. After the electrophoretic transfer of the proteins to PVDF membranes (Millipore, IPVH00010), nonspecific binding was blocked by incubation with 5% skim milk. The membranes were then incubated with the following primary antibodies: Influenza A NP Polyclonal Antibody (invitrogen, #PA5-32242, 1:2000), Anti-SARS-CoV-1/2 NP Antibody (Sigma-Aldrich, ZMS1075, 1:2000), Anti-Mumps NP antibody (Abcam, ab9880, 1:2000), Anti-Vaccinia virus antibody (Abcam, ab35219, 1:2000), Anti-HSV-1 ICP8 Major DNA binding protein antibody (Abcam, ab20194, 1:2000), anti-ZBP1 (AdipoGen, AG-20B-0010, 1:2000), and anti-β-actin (Proteintech, 66009-1- IG, 1:5000) antibodies. The membranes were then washed and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000 dilution; Jackson Immuno Research Laboratories, anti-rabbit [111-035-047], anti-mouse [315-035-047] antibodies) for 1 h. The proteins were visualized using Luminata Forte Western HRP Substrate (Millipore, WBLUF0500), and the membranes were analyzed using Amersham ImageQuant 800 UV. Images were analyzed by ImageJ. The relative intensity of protein band is quantified by Cytiva ImageQuant™ TL.
In vivo study of ginseng extracts reduce survival in IAV infection
Age- and sex-matched, 6- to 8-week-old wild-type and Zbp1–/– mice were used for infections. For IAV PR8 infection, mice were anaesthetized with 250 mg/kg avertin and then infected intranasally with IAV PR8 in 50 μl PBS containing around 3.04 × 103 PFU. Infected mice were monitored over a period of 14 days for survival. Korean Red ginseng extracts are treated orally 30 mg/kg/day for 7 days before virus infection and after 14 days of virus infection. Lung samples collected at 5 days after infection are fixed at NBF and then H&E staining were conducted through LabCore Korea. The specimens were examined utilizing a DMi8 Inverted microscope (Leica).
Results
Korean Red ginseng lowers viral protein expression in BMDMs infected with Influenza A virus
To evaluate the efficacy of Korean Red ginseng in viral clearance against a spectrum of DNA and RNA viruses. Prior to assessing its antiviral properties, the toxicity of Korean Red ginseng on host cells was examined to ensure safe experimental conditions. Wild-type (WT) bone marrow-derived macrophages (BMDMs) (Fig. S1A-B) and A549 (Fig. S1C-D) were treated with varying concentrations of Korean Red ginseng. The concentrations ranged from 25 μg/ml to 2.5 mg/ml, with a tenfold increase between each concentration. Cell viability was assessed after 24 h of treatment. The results revealed no significant difference in cell death between concentrations of 25 μg/ml and 250 μg/ml in both WT BMDMs and A549. However, a notable increase in cell death was observed at concentrations of 25 μg/ml and 2.5 mg/ml in WT BMDMs. Based on these findings, 250 μg/ml was selected as the experimental condition for further investigations (Fig. S1A-D).
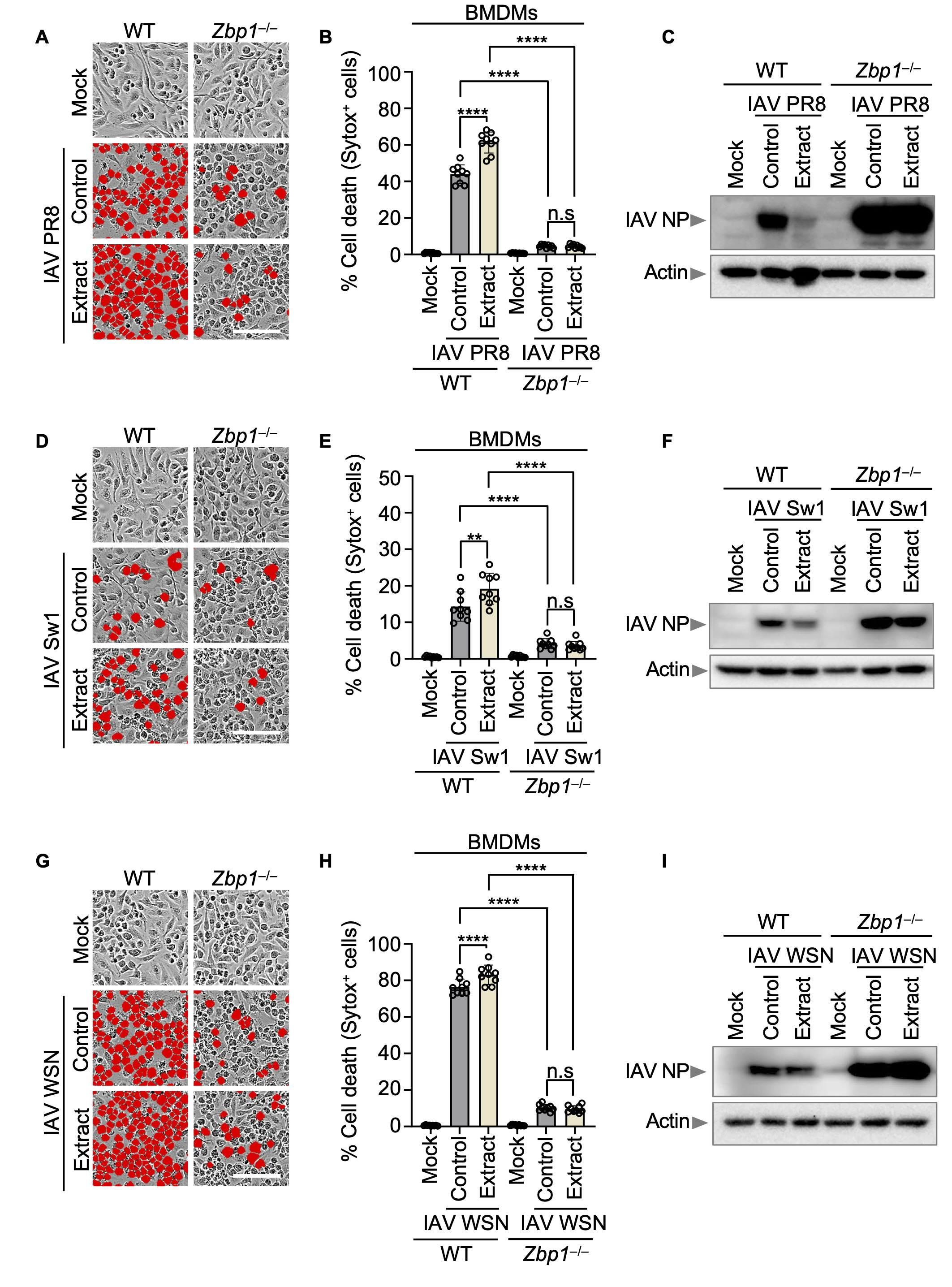
Among various viruses studied, Korean Red ginseng demonstrated significant efficacy against the influenza virus, particularly in terms of viral clearance (Lee et al., 2014a; Yoo et al., 2012a, 2012b). Initially, WT BMDMs were exposed to the Influenza A virus A/Puerto Rico/8/34 (PR8) at an MOI of 10. Following this, fetal bovine serum (FBS) was introduced one hour later, and subsequently, Korean Red ginseng extracts were administered at a concentration of 250 μg/ml. The incubation period lasted for 30 h. Observations indicated a higher rate of BMDMs cell death in the group treated with Korean Red ginseng compared to control group that was infected with the virus alone (Fig. 1A-B). However, intriguingly, despite the increased cell death, the expression level of viral protein in the Korean Red ginseng-treated group was notably lower compared to the control group infected solely with the virus (Fig. 1C-D). This suggests that while Korean Red ginseng induced cell death in virus infected cells, it simultaneously lowered the expression level of viral protein, ultimately contributing to its clearance.
Korean Red ginseng specifically shows the efficacy of viral protein expression against influenza viruses
To investigate the efficacy against RNA viruses, human coronavirus (HCoV-NL63; strain: NL63) and mumps virus (MuV; strain: MuV/Iowa.US/2006), were introduced to A549 at an MOI of 10. Following this, FBS was added one hour later to inactivate virus, with Korean Red ginseng treatment commencing another hour after that. Korean Red ginseng extracts was administered at a concentration of 250 μg/ml, and the incubation period lasted for 30 h. For evaluating the results, both host cell death and viral protein expression levels were compared between the Korean Red ginseng-treated groups and the control groups. However, no significant difference was observed between them (Fig. S2A-F). As a result, it was concluded that Korean Red ginseng did not exhibit efficacy of viral protein expression against these RNA viruses.
To investigate the potential virus clearance efficacy of Korean Red ginseng against DNA viruses, an experiment was conducted under conditions identical to the previous RNA virus experiments. Initially, WT BMDMs and Zbp1–/– BMDMs were infected with herpes simplex virus 1 (HSV-1; strain: HF) and vaccinia virus (VACV; strain: Western Reserve) at an MOI of 10. Subsequently, FBS was added one hour later, followed by the administration of 250 μg/ml of Korean Red ginseng extracts. The cells were then incubated for 30 h, respectively. The results demonstrated that in WT BMDMs, viral nucleoprotein (NP) expression was comparable between the control and Korean red ginseng-treated groups for both HSV-1 and VACV. Similarly, in Zbp1–/– BMDMs, the levels of viral NP expression were consistent with those observed in WT BMDMs (Fig. S3A-F). These findings indicate that ZBP1-mediated cell death does not play a significant role in the response to HSV-1 or VACV infection. This suggests that Korean Red ginseng did not demonstrate efficacy of viral expression against these DNA viruses under the experimental conditions.
ZBP1 is as a crucial innate immune sensor of Influenza A virus
ZBP1, also known as the DNA-dependent activator of interferon regulatory factor (DAI), is a critical non-NLR family inflammasome sensor integral to viral detection. This cytoplasmic sensor is essential for the recognition and response to viral pathogens, particularly IAV (Karki et al., 2022; Kuriakose et al., 2016; Thapa et al., 2016). Upon binding to viral RNA, ZBP1 initiates a signaling cascade resulting in the activation of inflammatory cytokines, including type I interferons (Karki et al., 2022). This mechanism involves the formation of inflammasomes within primary murine BMDMs. Inflammasome activation subsequently leads to the production of pro-inflammatory cytokines, which are crucial for orchestrating an effective antiviral response. Furthermore, the ability of ZBP1 is to mediate programmed cell death pathways, such as PANoptosis, highlights its significant role in controlling viral infections and restricting viral dissemination (Banoth et al., 2020; Karki et al., 2022; Kesavardhana et al., 2020; Lee et al., 2021b; Oh & Lee, 2023).
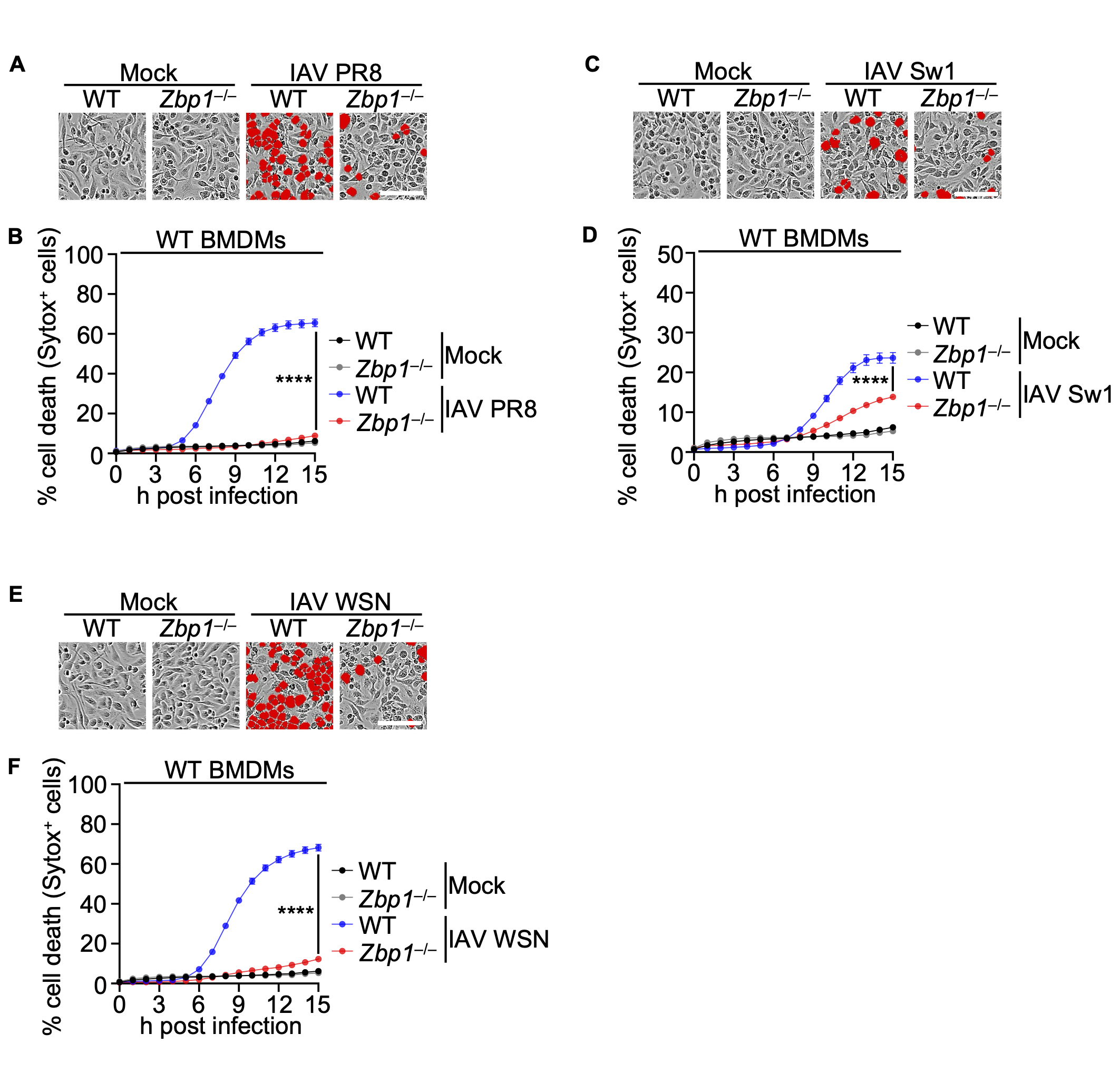
Subsequently, in our investigation, we examined the impact of ZBP1 deficiency on IAV infection by infecting Zbp1–/– BMDMs alongside WT BMDMs with IAV strains. Before that, among the various strains of Influenza A virus, PR8, Switzerland/2013, and WSN were selected as criteria for causing high host cell death in WT BMDMs. Results confirmed a significant reduction in cell death in Zbp1–/– BMDMs compared to their WT BMDMs (Fig. 2A-F).
ZBP1 and Korean Red ginseng induce cell death thereby it reduces various influenza viral protein expression
To investigate the relationship between Korean Red ginseng and ZBP1 in influenza virus infection. we infected IAV 3 strains (PR8, WSN, and Switzerland/2013) in both WT BMDMs and Zbp1–/– BMDMs at a MOI of 10. One-hour post-infection, FBS was added to inhibit viral entry, followed by the administration of Korean Red ginseng extract at a concentration of 250 μg/ml. Both cells were then incubated for an additional 30 h. In WT BMDMs, Korean Red ginseng treatment group resulted in a marked increase in cell death compared to the control group, while the expression level of the influenza A viral NP was notably reduced. Conversely, in Zbp1–/– BMDMs, both the virus-only and virus-Korean Red ginseng treated groups demonstrated significantly lower levels of cell death relative to WT BMDMs. Additionally, NP expression was substantially higher in Zbp1–/– BMDMs than in WT BMDMs. We found that Korean Red ginseng is associated with ZBP1 in inducing cell death, thereby reducing the expression of various influenza viral proteins (Fig. 3A-I).
ZBP1 is required for host defense thereby it reduces the expression of influenza viral protein NP along with Korean Red ginseng
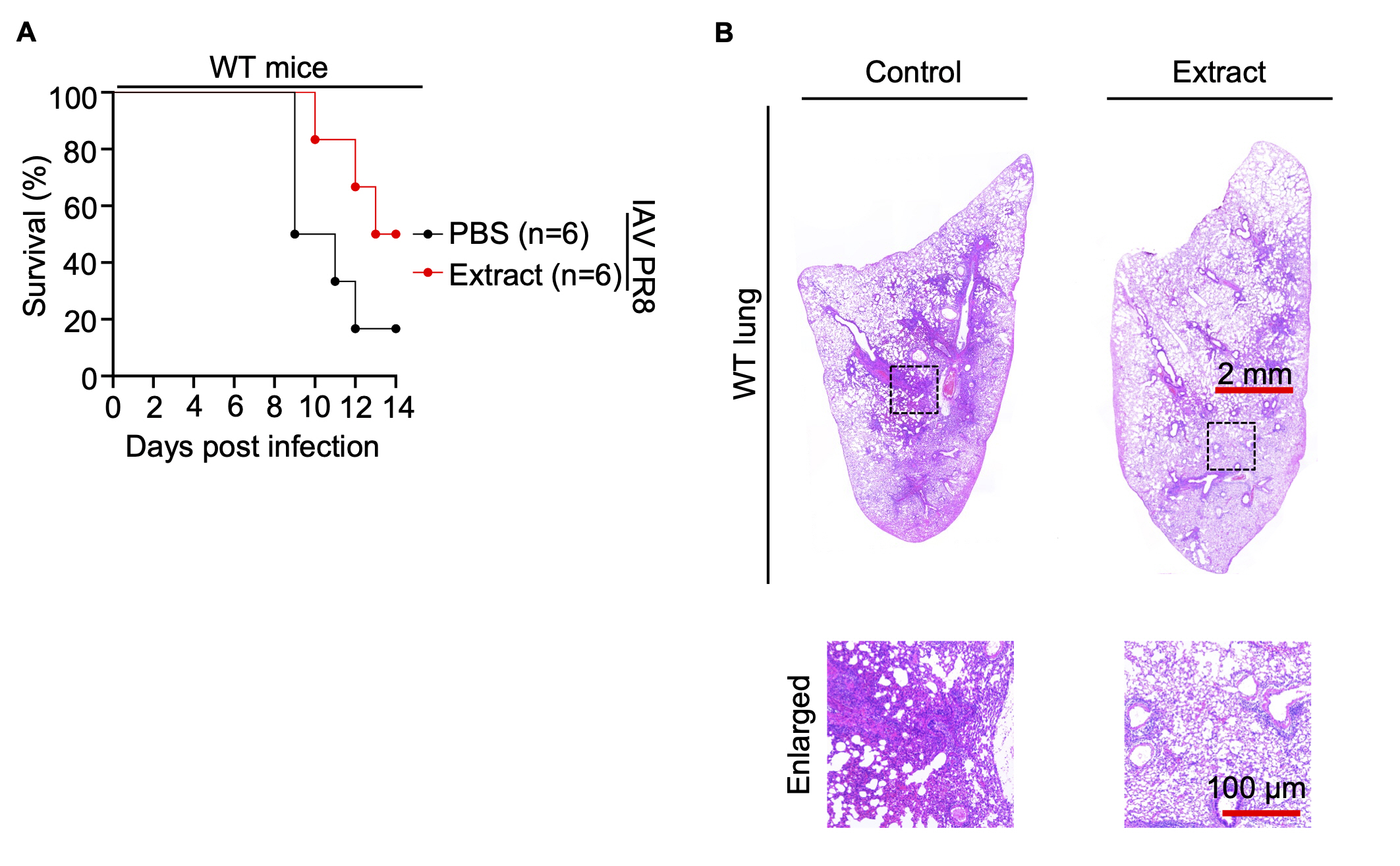
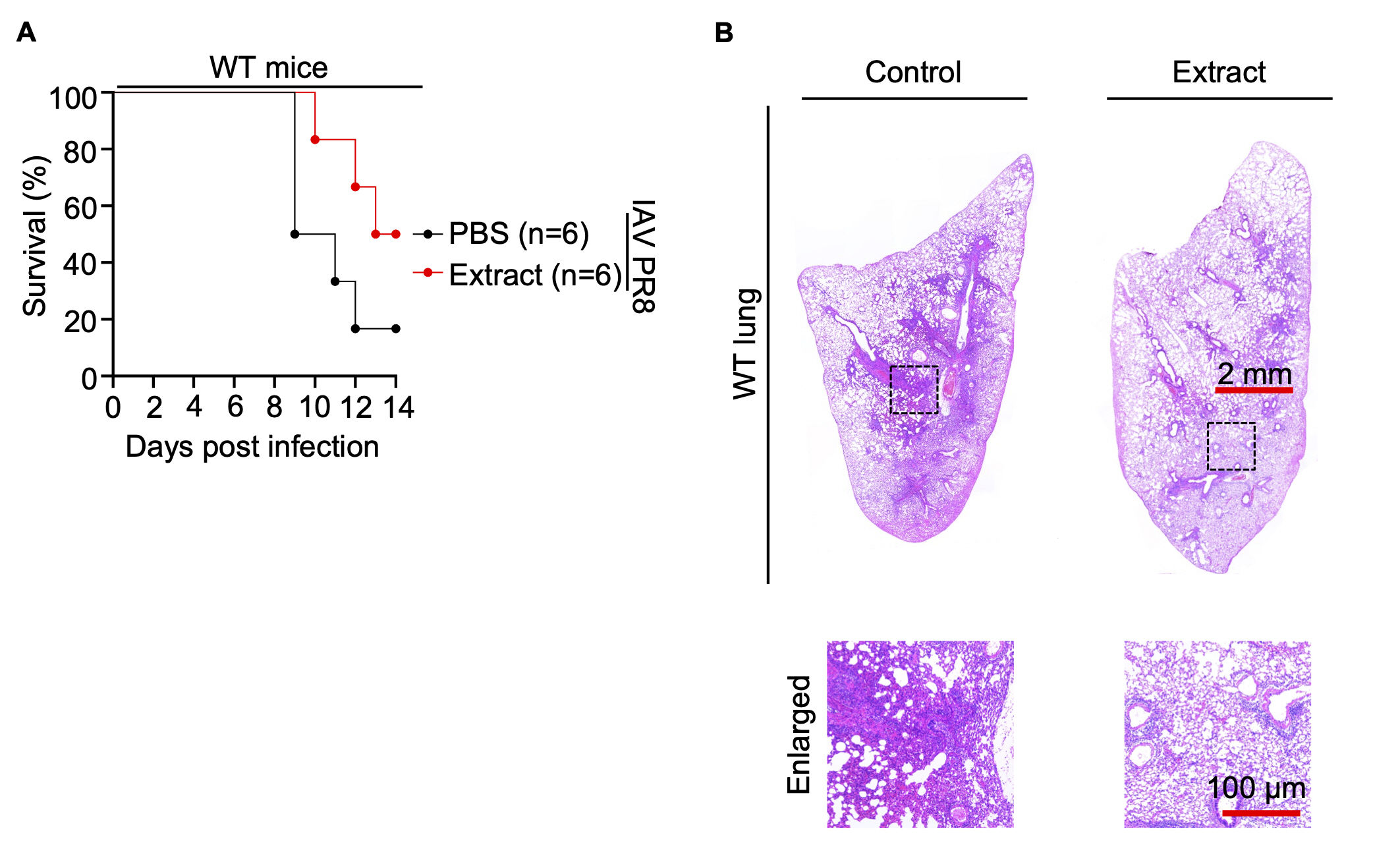
Subsequently, WT mice and Zbp1–/– mice were administered Korean Red ginseng for a duration of 7 days, after which they were inoculated with Influenza A virus PR8 on the 7th day. The mice were then closely monitored for changes in weight and overall survival over a span of 14 days. Experimental data show that in WT mice, treatment with Korean Red ginseng extracts improve survival compared to the control group treated with the virus alone (Fig. 4A). However, in Zbp1–/– mice, no significant difference in survival was observed between the virus-treated control group and Korean Red ginseng-treated group. These findings suggest that the beneficial effects of Korean Red ginseng on survival in IAV-infected mice are mediated through the ZBP1 pathway. In the absence of ZBP1, Korean Red ginseng fails to enhance survival, indicating that ZBP1 is essential for the activation of cell death pathways that contribute to the antiviral response facilitated by Korean Red ginseng. This result highlights the critical role of ZBP1 in the immunomodulatory effects of Korean Red ginseng and its potential as a therapeutic agent (Fig. S4A). Histopathological analysis of lung tissue from WT mice revealed that those administered red ginseng exhibited significantly reduced inflammation compared to those solely infected with the virus (Fig. 4B). On the other hand, histopathological analysis of the lungs in Zbp1–/– mice revealed that both the control and extract-treated groups exhibited similar patterns to those observed in the control of WT mice. This finding indicates that Zbp1–/– mice, when infected with the influenza virus, are unable to induce ZBP1-mediated cell death. Consequently, we concluded that administration of Korean Red ginseng enhancing ZBP1-mediated cell death did not cause additional observable changes in the Zbp1–/– mouse model (Fig. S4B).
Discussion
Red ginseng is derived from the roots of the Panax plant, it undergoes a specialized preparation process involving steaming and drying, enhancing its nutritional properties. Traditionally, Red ginseng used as a drug for viral disease or medicine to enhance the immune system (Kiefer & Pantuso, 2003). The presence of ginsenosides, unique active compounds, is thought to play a pivotal role in these immunomodulatory effects (Liu & Fan, 2018; Qu et al., 2011; Riaz et al., 2019). However, there were no medical evidence to prove the effectiveness. Current studies revealed that during viral infection, intake of ginseng is helpful to activating immune system and reducing viral replication by in vitro and in vivo experiments (Lee et al., 2014b, 2015). As research continues to unravel the complex interplay between ginseng and the immune system, the potential for ginseng-derived compounds to become recognized therapeutic agents in modern medicine becomes increasingly promising, blending ancient wisdom with contemporary scientific exploration (Liu & Fan, 2018; Riaz et al., 2019; Wang et al., 2021b; Xu et al., 2020; Yoon et al., 2023). However, there was still a lack of understanding regarding the molecular mechanisms involved in activating the immune system.
ZBP1 is a protein encoded by the ZBP1 gene. This protein plays a crucial role in the immune system, particularly in the detection of viral infections and the subsequent activation of antiviral responses. ZBP1 can detect viral nucleic acids, such as Z-RNA and Z-DNA, which are often produced during viral infections like IAV (Zhang et al., 2020). Z-RNA is a left-handed helical form of RNA distinguished by its unique zigzag sugar-phosphate backbone and alternating anti and syn conformations of nucleotides (Krall et al., 2023). This structural peculiarity sets it apart from the more prevalent right-handed RNA structures (Bartas et al., 2022). Notably, Z-RNA serves as a crucial molecular pattern recognized by proteins like ZBP1, initiating immune signalling cascades upon viral infection. As a cytosolic nucleic acid sensor, ZBP1 can recognize and bind to specific DNA structures, including Z-DNA, which can form during viral infections. The presence of Z-DNA or other abnormal DNA structures serves as a sign of viral activity and triggers ZBP1-mediated immune responses (Jiao et al., 2020; Lee et al., 2021c; Li et al., 2023).
Once ZBP1 detects IAV-derived RNA, it initiates a signaling cascade that activates the immune system's response. ZBP1 acts as an early warning signal, triggering downstream pathways that lead to the production of inflammatory cytokines and the activation of immune cells. PANoptosis is a unique form of programmed cell death characterized by the integration of pyroptosis, apoptosis, and necroptosis pathways. Pyroptosis involves inflammatory cell death mediated by inflammasomes, leading to cytokine release and cell lysis (Cookson & Brennan, 2001; Martinon et al., 2002). Apoptosis is a controlled form of cell death that occurs in response to various signals, resulting in cellular dismantling without inflammation (Boldin et al., 1996; Gross et al., 1999; Kim et al., 2005; Li et al., 1997, 1998; Luo et al., 1998; Muzio et al., 1996; Zou et al., 1997). Necroptosis is a regulated form of necrosis driven by receptor-interacting protein kinases (RIPK1 and RIPK3), leading to cell swelling and membrane rupture (Dhuriya & Sharma, 2018; Gong et al., 2019; Murphy et al., 2013; Nailwal & Chan, 2019; Newton et al., 2019; Sun et al., 2012; Zhao et al., 2012). PANoptosis combines aspects of these pathways to orchestrate a coordinated cell death response to diverse stimuli, ensuring effective clearance of damaged cells and pathogens (Karki et al., 2021b; Lee et al., 2020, 2021a; Oh & Lee, 2023; Wang et al., 2022b).
The role of ZBP1 in IAV detection and immunity is crucial for several reasons. Firstly, it provides an extra layer of defense against IAV by recognizing abnormal DNA structures that may be generated during viral replication. Secondly, the initiation of PANoptosis helps to rapidly contain and eradicate the infection, limiting the virus's ability to replicate and spread to neighboring cells and tissues. Finally, the activation of inflammatory responses by ZBP1 contributes to the overall immune defense against IAV and potentially other pathogens (Karki et al., 2022; Thomas et al., 2020). ZBP1 is also related to inflammasome. The inflammasome is a critical component of the innate immune system, serving as a sensor and signalling platform that detects danger signals and initiates inflammatory responses through the processing of pro-inflammatory cytokines (Yu et al., 2024). Its activation plays a crucial role in defending against pathogens, promoting tissue repair, and maintaining immune homeostasis.
In previous studies, there have been many studies to prove the antiviral activity of red ginseng against various viruses (Baek et al., 2010; Chandra Das et al., 2023; Cho et al., 2023; Lee et al., 2013; Pei et al., 2011; Yang et al., 2018). In particular, there have been studies that have investigated whether taking red ginseng before and after an influenza virus infection can help people overcome viral infection. Korean Red ginseng extracts increase the immunomodulatory cytokines and pro-inflammatory cytokines during different strains of influenza virus infection in in-vitro experiments (Lee et al., 2014a; Park et al., 2014; Yoo et al., 2012a, 2012b). And on the in vivo experiments, ginseng treated mice group resulted in higher survival than control groups during infection of IAV (Kim et al., 2016, 2011). However, most of the existing studies have only shown that the treatment of cells or mice with red ginseng can inhibit viral replication. There is lack of studies about molecular mechanisms that how to regulate the immune activation.
In this study, Korean Red ginseng extracts treatment during IAV infection increase the cell death rate and lower the viral expression of cell (Fig. 1). When infected with IAV, to protect the host, innate immunity is activated by sensing the viral genome by ZBP1. As a part of innate immunity, programmed cell death occurs to reduce the amount of viral particle and prevent the viral replication. Thus, in the absence of ZBP1, cell death for host defence does not occur. This is not specific to IAV A/Puerto Rico/8/34 but also to various strains of IAV, including A/Switzerland/9715293/2013, and A/WSN/1933 (Fig. 2). And induced cell death reduces the number of viral proteins in cell (Fig. 3). Therefore, Korean Red ginseng is used as immune enhancer of ZBP1 mediated cell death process. Applying this result to mice as well, wild-type (WT) mice treated with Korean Red ginseng extracts group resulted higher survival than control group. But in the Zbp1–/– mice group, there are no significant difference in between control group (Fig. 4 and Fig. S4). In the absence of ZBP1, it does not induce programmed cell death. Therefore, the treatment with Korean Red ginseng did not eliminate the virus, as it could not cause cell death due to the absence of ZBP1, as demonstrated in the in vivo experiment.
This study elucidates an indirect connection between ZBP1 and Korean Red ginseng extracts. ZBP1, sensor of IAV viral DNA, modulate the cell death during IAV infection. And Korean Red ginseng extracts effectively reduce the viral protein of IAV. From this, we can infer that ZBP1 and Korean Red ginseng extracts interact with each other to defend the host during IAV infection. Then Korean Red ginseng extracts can be additional immune enhancer in context of IAV infection. Our findings establish a foundation for further investigation into red ginseng-derived compounds or analogs with enhanced immunomodulatory properties. The intricate relationship between red ginseng and the ZBP1 protein presents promising opportunities for future research and therapeutic innovation. A primary focus for subsequent studies will be to thoroughly elucidate the mechanisms by which Korean Red ginseng interacts with ZBP1 and influences the programmed cell death pathway, a complex process involving multiple cellular mechanisms.
Advanced molecular techniques will be crucial in uncovering the precise pathways through which Korean Red ginseng modulates ZBP1 function, thereby facilitating the development of targeted red ginseng-derived compounds. Moreover, the role of Korean Red ginseng in regulating PANoptosis, particularly in the context of viral infections, warrants further investigation. Future research should explore potential synergistic interactions between Korean Red ginseng and conventional antiviral therapies to enhance therapeutic efficacy and minimize adverse effects.
Acknowledgments
We extend our gratitude to members of the Lee’s Viral Immunology Lab for their valuable insights, comments, and suggestions. We also thank UCRF (UNIST Central Research Facility), especially the IVRC (In vivo Research Center) for the use of their equipment. We thank Atsushi Kawaguchi (University of Tsukuba) for A549 cells and Man-Seong Park (Korea University) for the gift of the Influenza A virus A/Puerto Rico/8/34 (H1N1, PR8), A/Switzerland/9715293/2013 (H3N2, Switzerland/2013), and A/WSN/1933 (H1N1, WSN). We acknowledge Korea Ginseng Corporation (KGC) for the Korean Red ginseng extracts.
Author Contributions
J.O., H.K., and S.L. conceived the study's conceptual framework; J.O., H.K., and S.L. formulated the research methodology; J.O., H.K., J.L., S.K., and S.S. conducted the experiments; J.O., H.K., J.L., S.K., S.S., Y.K., S.P., and S.L. performed the data analysis; J.O., H.K., and S.L. composed the manuscript; and S.L. secured funding and provided overall supervision.
Conflict of Interest
The authors declare no potential conflicts of interest.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (2022R1C1C1007544, 2024M3A9H5043152 to S.L.), a grant from the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea under the Korea Health Technology R&D Project (RS-2022-KH128422(HV22C015600) to S.L.), and the Institute for Basic Science (IBS), Republic of Korea (IBS-R801-D9-A09 to S.L.). Moreover, this work was supported by The Circle Foundation (Republic of Korea) through the selection of the UNIST Pandemic Treatment Research Center as the 2023 The Circle Foundation Innovative Science Technology Center (2023 TCF Innovative Science Project-01 to S.L.). Additionally, research funds were granted from the Republic of Korea's National Institute of Health (2022-NI-072-00, 2022-NI-040-01 to S.L.), the Ulsan National Institute of Science & Technology (UNIST) (1.220112.01, 1.220107.01 to S.L.), The Korean Society of Ginseng 2023 (S.L.) and Yuhan Corporation (S.L.).
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.71150/jm.2409007.
Fig. S1.
250 μg/mL of Korean Red ginseng is proper concentration that does not induce cell death in BMDMs.
A Cell death in wild-type (WT) bone marrow-derived macrophages (BMDMs) after Korean Red ginseng extracts treatment for 30h with does dependent manner. Red indicates dead cells. Scale bar, 100μm. B Quantification of the cell death in panel A. Data are mean±s.e.m. ***P<0.001 (one-way ANOVA with Dunnett’s multiple comparisons test; n=4 from three biologically independent samples). C Cell death in A549 treated with Korean Red ginseng extracts treatment for 30h with does dependent manner. Red indicates dead cells. Scale bar, 100μm. D Quantification in the cell death in panel C. Data are mean±s.e.m. ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples).
jm-2409007-Supplementary-Fig-S1.pdf
Fig. S2.
Korean Red ginseng extracts induce no changes of cell death and viral protein expression during other RNA viral infections; Human coronavirus-NL63 and Mumps virus.
A Cell death in A549 after HCoV-NL63 infection and Korean Red ginseng extracts treatment for 30h. Red indicates dead cells. Scale bar, 100μm. Images are representative of at least three independent experiments. B Quantification of the cell death in panel A. Data are mean±s.e.m. ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). C Immunoblot analysis of HCoV-NL63 NP expression in A549 after HCoV-NL63 infection and Korean Red ginseng extracts treatment. Data are representative of at least three independent experiments. D Cell death in A549 after mumps virus (MuV) infection and Korean Red ginseng extracts treatment for 30h. Red indicates dead cells. Scale bar, 100μm. Images are representative of at least three independent experiments. E Quantification of the cell death in panel D. Data are mean±s.e.m. ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). F Immunoblot analysis of MuV NP expression in A549 after MuV infection and Korean Red ginseng extracts treatment. Data are representative of at least three independent experiments.
jm-2409007-Supplementary-Fig-S2.pdf
Fig. S3.
Korean Red ginseng does not reduce the expression of viral protein during DNA virus infection; Herpes Simplex virus-1 or Vaccinia virus.
A Cell death in wild-type (WT) and Zbp1–/– bone marrow-derived macrophages (BMDMs) after herpes simplex virus-1 (HSV-1) infection and Korean Red ginseng extracts treatment for 30h. Red indicates dead cells. Scale bar, 100μm. Images are representative of at least three independent experiments. B Quantification of the cell death in panel A. Data are mean±s.e.m. ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). C Immunoblot analysis of HSV-1 ICP8 and actin expression in WT and Zbp1–/– BMDMs after HSV-1 infection and Korean Red ginseng extracts treatment for 30h. Data are representative of at least three independent experiments. D Cell death in wild-type (WT) and Zbp1–/– bone marrow-derived macrophages (BMDMs) after vaccinia virus (VACV) infection and Korean Red ginseng extracts treatment for 30h. Red indicates dead cells. Scale bar, 100μm. Images are representative of at least three independent experiments. E Quantification of the cell death in panel D. Data are mean±s.e.m. *P<0.05 (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). F Immunoblot analysis of VACV and actin expression in WT and Zbp1–/– BMDMs after VACV infection and Korean Red ginseng extracts treatment for 30h. Data are representative of at least three independent experiments.
jm-2409007-Supplementary-Fig-S3.pdf
Fig. S4.
Korean Red ginseng cannot counteract influenza virus due to the absence of ZBP1.
A The survival of 6- to 8-week-old Zbp1–/– mice treated with PBS (n=6) or Korean Red ginseng extracts (n=7) every day for 7 days before IAV PR8 infection and for 14 days after IAV PR8 infection. P values for survival of Korean Red ginseng extracts group versus PBS group are shown in the key. P=0.6526 (log-rank (Mantel-Cox) test). B Lung tissue sections of Zbp1–/– mice were stained with hematoxylin and eosin. Scale bar, 2mm (top), 100μm (bottom).
jm-2409007-Supplementary-Fig-S4.pdf
Fig. 1.
Korean Red ginseng extracts reduce the expression of viral protein NP with inducing cell death in influenza A virus infection.
(A) Cell death in wild-type (WT) bone marrow-derived macrophages (BMDMs) after IAV PR8 infection and Korean Red ginseng extracts treatment for 30 h. Red indicates dead cells. Scale bar, 100 μm. Images are representative of at least three independent experiments. (B) Quantification of the cell death in panel A. Data are mean±s.e.m. ****P<0.0001 (one-way ANOVA with Dunnett’s multiple comparisons test; n=4 from four biologically independent samples). (C) Immunoblot analysis of IAV NP expression in BMDMs after IAV PR8 infection and Korean Red ginseng extracts treatment. Data are representative of at least three independent experiments. (D) Quantification of relative intensity in panel C. The value of intensity is expression of IAV NP relative to actin in panel C. Data are mean±s.e.m. *P<0.05 (one-way ANOVA with Dunnett’s multiple comparisons test; n=3 from three biologically independent samples).
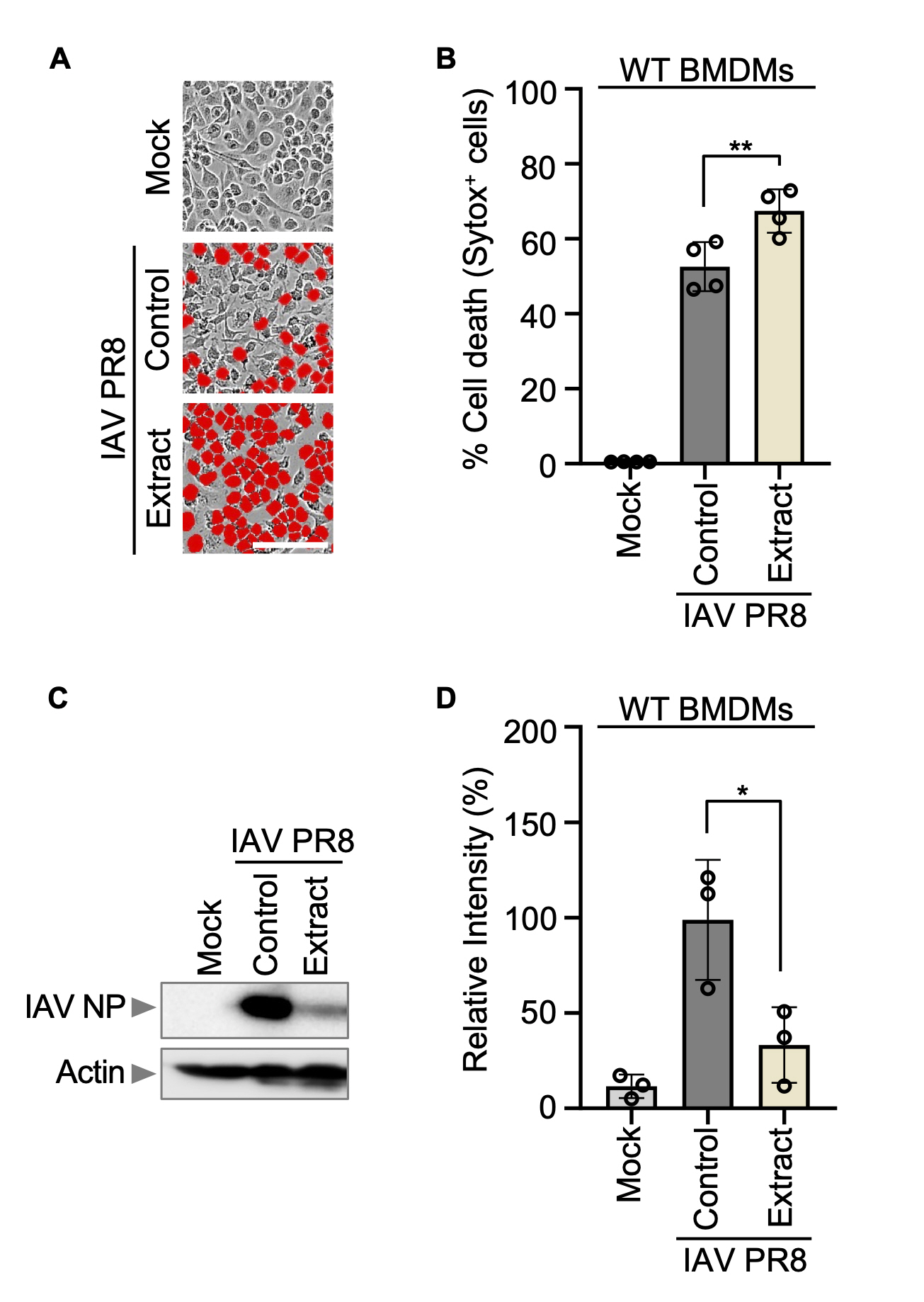
Fig. 2.
Cell death induced by IAV infection depends on ZBP1.
(A) Cell death in wild-type (WT) or Zbp1–/– bone marrow-derived macrophages (BMDMs) after IAV PR8 infection for 15 h. Red indicates dead cells. Scale bar, 100 μm. Images are representative of at least three independent experiments. (B) Quantification of the cell death in panel A. Data are mean±s.e.m. ****P<0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). (C) Cell death in WT or Zbp1–/– BMDMs after IAV Switzerland/2013 infection for 15 h. Red indicates dead cells. Scale bar, 100 μm. Images are representative of at least three independent experiments. (D) Quantification of the cell death in panel C. Data are mean±s.e.m. ****P<0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples). (E) Cell death in WT or Zbp1–/– BMDMs after IAV WSN infection for 15 h. Red indicates dead cells. Scale bar, 100 μm. Images are representative of at least three independent experiments. (F) Quantification of the cell death in panel E. Data are mean±s.e.m. ****P<0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test; n=9 from three biologically independent samples).
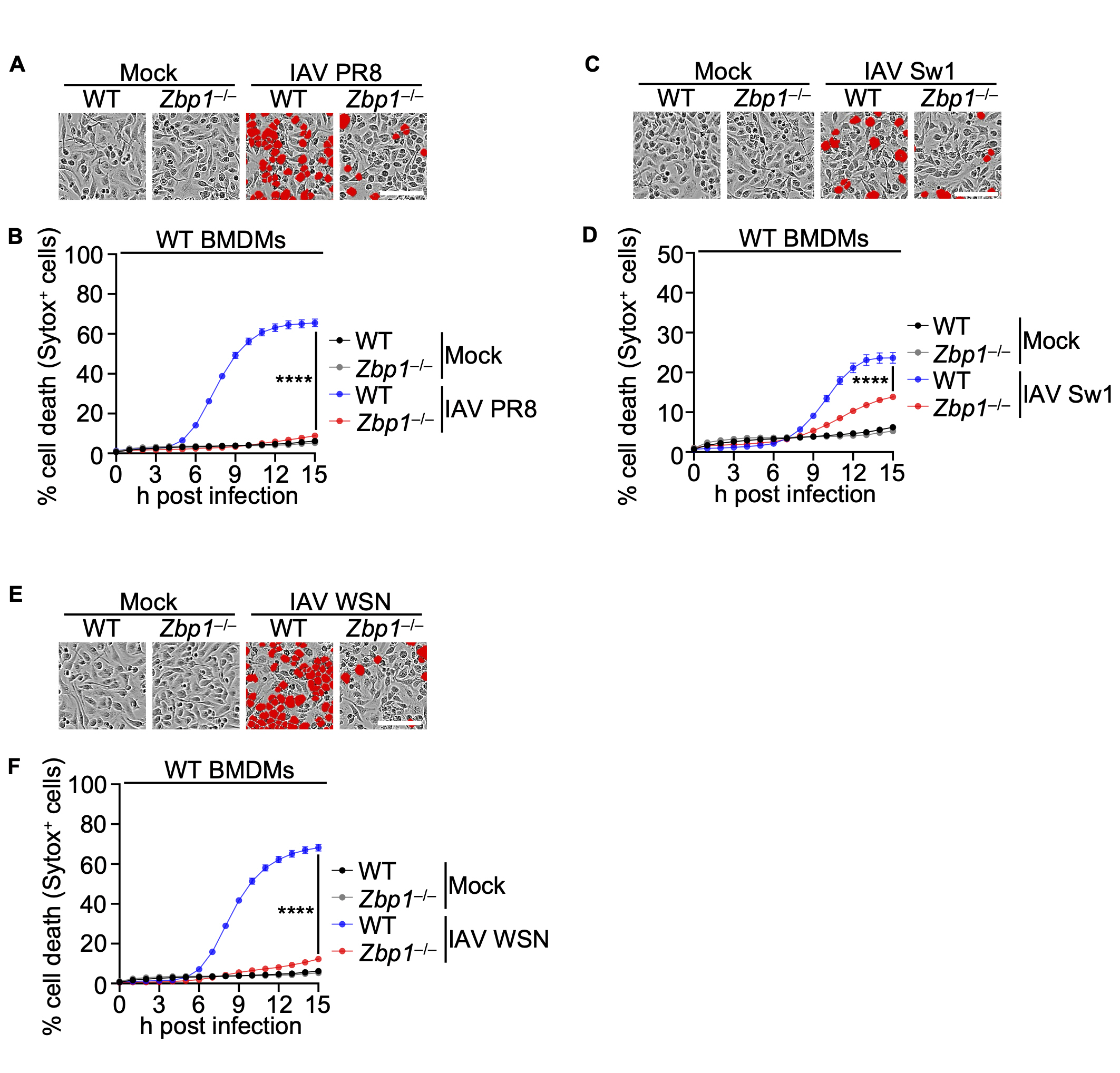
Fig. 3.
ZBP1 reduces the amount of IAV viral protein expression during IAV infection with Korean Red ginseng extracts.
(A) Cell death in wild-type (WT) and Zbp1–/– bone marrow-derived macrophages (BMDMs) after IAV PR8 infection and Korean Red ginseng extracts treatment for 30 h. Red indicates dead cells. Scale bar, 100 μm. (B) Quantification of the cell death in panel A. Data are mean±s.e.m. ****P<0.0001; ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from four biologically independent samples). (C) Immunoblot analysis of IAV NP and actin expression in WT and Zbp1–/– BMDMs after IAV PR8 infection and Korean Red ginseng extracts treatment for 30 h. Data are representative of at least three independent experiments. (D) Cell death in WT and Zbp1–/– BMDMs after IAV Switzerland/2013 infection and Korean Red ginseng extracts treatment for 30h. Red indicates dead cells. Scale bar, 100 μm. (E) Quantification of the cell death in panel C. Data are mean±s.e.m. ****P<0.0001; **P<0.01; ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from four biologically independent samples). (F) Immunoblot analysis of IAV NP and actin expression in WT and Zbp1–/– BMDMs after IAV Switzerland/2013 infection and Korean Red ginseng extracts treatment for 30 h. Data are representative of at least three independent experiments. (G) Cell death in WT and Zbp1–/– BMDMs after IAV WSN infection and Korean Red ginseng extracts treatment for 30 h. Red indicates dead cells. Scale bar, 100 μm. (H) Quantification of the cell death in panel G. Data are mean±s.e.m. ****P<0.0001; ns, not significant (one-way ANOVA with Dunnett’s multiple comparisons test; n=9 from four biologically independent samples). (I) Immunoblot analysis of IAV NP and actin expression in WT and Zbp1–/– BMDMs after IAV WSN infection and Korean Red ginseng extracts treatment for 30 h. Data are representative of at least three independent experiments.

Fig. 4.
Korean Red ginseng reduces mortality against IAV PR8 in WT mice.
(A) Survival of 6- to 8-week-old wild-type (WT) mice treated with PBS (n=6) or Korean Red ginseng extracts (n=6) every day for 7 days before IAV PR8 infection and for 14 days after IAV PR8 infection. P values for survival of Korean Red ginseng extracts group versus PBS group are shown in the key. P=0.1069 (log-rank (Mantel-Cox) test). (B) Lung tissue sections of WT mice were stained with hematoxylin and eosin. Scale bar, 2 mm (top), 100 μm (bottom).
References
- Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. 2006. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 46(2): 187–197.ArticlePubMed
- Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, et al. 2011. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 195(6): 931–942.ArticlePubMedPMCPDF
- Baek SH, Lee JG, Park SY, Bae ON, Kim DH, et al. 2010. Pectic polysaccharides from Panax ginseng as the antirotavirus principals in ginseng. Biomacromolecules. 11(8): 2044–2052.ArticlePubMed
- Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, et al. 2020. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem. 295(52): 18276–18283.ArticlePubMedPMC
- Bao L, Cai X, Wang J, Zhang Y, Sun B, et al. 2016. Anti-fatigue effects of small molecule oligopeptides isolated from Panax ginseng CA Meyer in mice. Nutrients. 8(12): 807.ArticlePubMedPMC
- Bartas M, Slychko K, Brázda V, Červeň J, Beaudoin CA, et al. 2022. Searching for new Z-DNA/Z-RNA binding proteins based on structural similarity to experimentally validated Zα domain. Int J Mol Sci. 23(2): 768.ArticlePubMedPMC
- Boldin MP, Goncharov TM, Goltseve YV, Wallach D. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell. 85(6): 803–815.ArticlePubMed
- Chandra Das R, Ratan ZA, Rahman MM, Runa NJ, Mondal S, et al. 2023. Antiviral activities of ginseng and its potential and putative benefits against monkeypox virus: A mini review. J Ginseng Res. 47(6): 687–693.ArticlePubMedPMC
- Cho YK, Kim JE. 2017. Effect of Korean Red Ginseng intake on the survival duration of human immunodeficiency virus type 1 patients. J Ginseng Res. 41(2): 222–226.ArticlePubMedPMC
- Cho YK, Kim JE, Lee J. 2023. Korean Red Ginseng slows coreceptor switch in HIV-1 infected patients. J Ginseng Res. 47(1): 117–122.ArticlePubMedPMC
- Choi JY, Woo TS, Yoon SY, Choi YJ, Ahn HS, et al. 2011. Red ginseng supplementation more effectively alleviates psychological than physical fatigue. J Ginseng Res. 35(3): 331.ArticlePubMedPMC
- Cookson BT, Brennan MA. 2001. Pro-inflammatory programmed cell death. Trends Microbiol. 9(3): 113–114.ArticlePubMed
- Dhuriya YK, Sharma D. 2018. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 15: 1–9.ArticlePubMedPMCPDF
- Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 73(4): 1907–1916.ArticlePubMedPMCPDF
- Gong Y, Fan Z, Luo G, Yang C, Huang Q, et al. 2019. The role of necroptosis in cancer biology and therapy. Mol Cancer. 18: 1–17.ArticlePubMedPMCPDF
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, et al. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 274(2): 1156–1163.ArticlePubMed
- Ha SC, Kim D, Hwang HY, Rich A, Kim YG, et al. 2008. The crystal structure of the second Z-DNA binding domain of human DAI (ZBP1) in complex with Z-DNA reveals an unusual binding mode to Z-DNA. Proc Natl Acad Sci USA. 105(52): 20671–20676.ArticlePubMedPMC
- Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, et al. 2020. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 580(7803): 391–395.ArticlePubMedPMCPDF
- Jung JH, Kang IG, Kim DY, Hwang YJ, Kim ST. 2013. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 37(2): 167.ArticlePubMedPMC
- Karki R, Lee S, Mall R, Pandian N, Wang Y, et al. 2022. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci Immunol. 7(74): eabo6294.ArticlePubMedPMC
- Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, et al. 2021a. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 184(1): 149–168.ArticlePubMedPMC
- Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RS, et al. 2021b. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 37(3): 109858.ArticlePubMedPMC
- Kenarova B, Neychev H, Hadjiivanova C, Petkov VD. 1990. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn J Pharmacol. 54(4): 447–454.ArticlePubMed
- Kesavardhana S, Malireddi RS, Burton AR, Porter SN, Vogel P, et al. 2020. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. 295(24): 8325–8330.ArticlePubMedPMC
- Kiefer D, Pantuso T. 2003. Panax ginseng. Am Fam Physician. 68(8): 1539–1542.PubMed
- Kim HE, Du F, Fang M, Wang X. 2005. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci USA. 102(49): 17545–17550.ArticlePubMedPMC
- Kim JY, Germolec DR, Luster MI. 1990. Panax ginseng as a potential immunomodulator: studies in mice. Immunopharmacol Immunotoxicol. 12(2): 257–276.ArticlePubMed
- Kim H, Jang M, Kim Y, Choi J, Jeon J, et al. 2016. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J Pharm Pharmacol. 68(3): 406–420.ArticlePubMedPDF
- Kim KA, Jung JH, Choi YS, Kim ST. 2024. Ginsenoside Re protects rhinovirus-induced disruption of tight junction through inhibition of ROS-mediated phosphatases inactivation in human nasal epithelial cells. Heliyon. 10(5): e27688.ArticlePubMedPMC
- Kim WY, Kim JM, Han SB, Lee SK, Kim ND, et al. 2000. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 63(12): 1702–1704.ArticlePubMed
- Kim JY, Kim HJ, Kim HJ. 2011. Effect of oral administration of Korean red ginseng on influenza A (H1N1) virus infection. J Ginseng Res. 35(1): 104–110.Article
- Kim DY, Yang WM. 2011. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 136(1): 230–235.ArticlePubMed
- Krall JB, Nichols PJ, Henen MA, Vicens Q, Vögeli B. 2023. Structure and formation of Z-DNA and Z-RNA. Molecules. 28(2): 843.ArticlePubMedPMC
- Kuriakose T, Man SM, Subbarao Malireddi R, Karki R, Kesavardhana S, et al. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 1(2): aag2045.ArticlePubMedPMC
- Lamkanfi M, Dixit VM. 2010. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 8(1): 44–54.ArticlePubMed
- Lee S, Channappanavar R, Kanneganti TD. 2020. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 41(12): 1083–1099.ArticlePubMedPMC
- Lee JH, Cho SH. 2011. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 133(2): 810–817.ArticlePubMed
- Lee S, Cho HJ, Ryu JH. 2021a. Innate immunity and cell death in Alzheimer's disease. ASN Neuro. 13: 17590914211051908.ArticlePubMedPMCPDF
- Lee S, Hirohama M, Noguchi M, Nagata K, Kawaguchi A. 2018. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J Virol. 92(14): e00396–18.ArticlePubMedPMCPDF
- Lee JS, Hwang HS, Ko EJ, Lee YN, Kwon YM, et al. 2014a. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 6(2): 517–529.ArticlePubMedPMC
- Lee S, Ishitsuka A, Kuroki T, Lin YH, Shibuya A, et al. 2021b. Arf6 exacerbates allergic asthma through cell-to-cell transmission of ASC inflammasomes. JCI Insight. 6(16): e139190.ArticlePubMedPMC
- Lee S, Ishitsuka A, Noguchi M, Hirohama M, Fujiyasu Y, et al. 2019. Influenza restriction factor MxA functions as inflammasome sensor in the respiratory epithelium. Sci Immunol. 4(40): eaau4643.ArticlePubMed
- Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, et al. 2021c. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 597(7876): 415–419.ArticlePubMedPMCPDF
- Lee JS, Ko EJ, Hwang HS, Lee YN, Kwon YM, et al. 2014b. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 34(1): 183–190.ArticlePubMedPMC
- Lee MH, Lee BH, Lee S, Choi C. 2013. Reduction of hepatitis A virus on FRhK‐4 cells treated with Korean red ginseng extract and ginsenosides. J Food Sci. 78(9): M1412–M1415.ArticlePubMed
- Lee JS, Lee YN, Lee YT, Hwang HS, Kim KH, et al. 2015. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients. 7(2): 1021–1036.ArticlePubMedPMC
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, et al. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91(4): 479–489.ArticlePubMed
- Li S, Zhang Y, Guan Z, Ye M, Li H, et al. 2023. SARS-CoV-2 Z-RNA activates the ZBP1-RIPK3 pathway to promote virus-induced inflammatory responses. Cell Res. 33(3): 201–214.ArticlePubMedPMCPDF
- Li B, Zhao J, Wang CZ, Searle J, He TC, et al. 2011. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 301(2): 185–192.ArticlePubMedPMC
- Li H, Zhu H, Xu CJ, Yuan J. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 94(4): 491–501.ArticlePubMed
- Liu Y, Fan D. 2018. Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model. Food Function. 9(11): 5513–5527.ArticlePubMed
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 94(4): 481–490.ArticlePubMed
- Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, et al. 2017. Sensing of viral and endogenous RNA by ZBP 1/DAI induces necroptosis. EMBO J. 36(17): 2529–2543.ArticlePubMedPMC
- Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 10(2): 417–426.ArticlePubMed
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, et al. 2013. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 39(3): 443–453.ArticlePubMed
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, et al. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 85(6): 817–827.ArticlePubMed
- Nailwal H, Chan FKM. 2019. Necroptosis in anti-viral inflammation. Cell Death Differ. 26(1): 4–13.ArticlePubMedPMCPDF
- Nakaya TA, Kita M, Kuriyama H, Iwakura Y, Imanishi J. 2004. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J Interferon Cytokine Res. 24(2): 93–100.ArticlePubMed
- Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, et al. 2019. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 574(7778): 428–431.ArticlePubMedPDF
- Oh S, Lee S. 2023. Recent advances in ZBP1-derived PANoptosis against viral infections. Front Immunol. 14: 1148727.ArticlePubMedPMC
- Oh S, Lee J, Oh J, Yu G, Ryu H, et al. 2023. Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. Cell Mol Immunol. 20(12): 1513–1526.ArticlePubMedPMCPDF
- Ota T, Fujikawa-yamamoto K, Zong ZP, Yamazaki M, Odashima S, et al. 1987. Plant-glycoside modulation of cell surface related to control of differentiation in cultured B16 melanoma cells. Cancer Res. 47(14): 3863–3867.ArticlePubMed
- Ota T, Maeda M, Odashima S, Ninomiya-Tsuji J, Tatsuka M. 1996. G1 phase-specific suppression of the Cdk2 activity by ginsenoside Rh2 in cultured murine cells. Life Sci. 60(2): PL39–PL44.ArticlePubMed
- Park EK, Choo MK, Han MJ, Kim DH. 2004. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 133(2): 113–120.ArticlePubMedPDF
- Park HM, Kim SJ, Kim JS, Kang HS. 2012. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 50(8): 2736–2741.ArticlePubMed
- Park JS, Park EM, Kim DH, Jung K, Jung JS, et al. 2009. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 209(1-2): 40–49.ArticlePubMed
- Park EH, Yum J, Ku KB, Kim HM, Kang YM, et al. 2014. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 38(1): 40–46.ArticlePubMedPMC
- Pei Y, Du Q, Liao PY, Chen ZP, Wang D, et al. 2011. Notoginsenoside ST-4 inhibits virus penetration of herpes simplex virus in vitro. J Asian Nat Prod Res. 13(06): 498–504.ArticlePubMed
- Placido D, Brown BA, Lowenhaupt K, Rich A, Athanasiadis A. 2007. A left-handed RNA double helix bound by the Zα domain of the RNA-editing enzyme ADAR1. Structure. 15(4): 395–404.ArticlePubMedPMC
- Popovich DG, Kitts DD. 2002. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophysics. 406(1): 1–8.ArticlePubMed
- Qian M, Yi L, Song-Lin L, Jie Y, Ping-Hu Z, et al. 2014. Chemical profiles and anticancer effects of saponin fractions of different polarity from the leaves of Panax notoginseng. Chin J Nat Med. 12(1): 30–37.ArticlePubMed
- Qu DF, Yu HJ, Liu Z, Zhang DF, Zhou QJ, et al. 2011. Ginsenoside Rg1 enhances immune response induced by recombinant Toxoplasma gondii SAG1 antigen. Vet Parasitol. 179(1-3): 28–34.ArticlePubMed
- Riaz M, Rahman NU, Zia-Ul-Haq M, Jaffar HZ, Manea R. 2019. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci Technol. 83: 12–30.Article
- Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. 2001. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 8(9): 761–765.ArticlePubMed
- Song JH, Choi HJ, Song HH, Hong EH, Lee BR, et al. 2014. Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J Ginseng Res. 38(3): 173–179.Article
- Sumiyoshi M, Sakanaka M, and Kimura Y. 2010. Effects of Red Ginseng extract on allergic reactions to food in Balb/c mice. J Ethnopharmacol. 132(1): 206–212.ArticlePubMed
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, et al. 2011. Red notoginseng: Higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 125(4): 1299–1305.ArticlePubMedPMC
- Sun L, Wang H, Wang Z, He S, Chen S, et al. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 148(1): 213–227.ArticlePubMed
- Sung H, Jung YS, Cho YK. 2009. Beneficial effects of a combination of Korean red ginseng and highly active antiretroviral therapy in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol. 16(8): 1127–1131.ArticlePubMedPMCPDF
- Sung H, Kang SM, Lee MS, Kim TG, Cho YK. 2005. Korean red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol. 12(4): 497–501.ArticlePubMedPMCPDF
- Tan S, Zhou F, Li N, Dong Q, Zhang X, et al. 2013. Anti-fatigue effect of ginsenoside Rb1 on postoperative fatigue syndrome induced by major small intestinal resection in rat. Biol Pharm Bull. 36(10): 1634–1639.ArticlePubMed
- Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, et al. 2016. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 20(5): 674–681.ArticlePubMedPMC
- Thomas PG, Shubina M, Balachandran S. 2020. ZBP1/DAI-dependent cell death pathways in influenza A virus immunity and pathogenesis. In Alternate Programmed Cell Death Signaling in Antiviral Host Defense, pp. 41–63, Springer.PDF
- Tode T, Kikuchi Y, Kita T, Hirata J, Imaizumi E, et al. 1993. Inhibitory effects by oral administration of ginsenoside Rh 2 on the growth of human ovarian cancer cells in nude mice. J Cancer Res Clin Oncol. 120(1-2): 24–26.ArticlePubMedPDF
- Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. 1998. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 246(3): 725–730.ArticlePubMed
- Wang Y, Karki R, Mall R, Sharma BR, Kalathur RC, et al. 2022a. Molecular mechanism of RIPK1 and caspase-8 in homeostatic type I interferon production and regulation. Cell Rep. 41(1): 111434.ArticlePubMedPMC
- Wang Y, Karki R, Zheng M, Kancharana B, Lee S, et al. 2021a. Cutting edge: caspase-8 is a linchpin in caspase-3 and gasdermin D activation to control cell death, cytokine release, and host defense during influenza A virus infection. J Immunol. 207(10): 2411–2416.ArticlePubMedPMCPDF
- Wang J, Li S, Fan Y, Chen Y, Liu D, et al. 2010. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. J Ethnopharmacol. 130(2): 421–423.ArticlePubMed
- Wang Z, Liu R, Chen L, Wang H, Zhou M, et al. 2021b. Pharmacokinetics of ginsenoside Rh2, the major anticancer ingredient of ginsenoside H dripping pills, in healthy subjects. Clin Pharmacol Drug Dev. 10(6): 669–674.ArticlePubMedPDF
- Wang Y, Pandian N, Han JH, Sundaram B, Lee S, et al. 2022b. Single cell analysis of PANoptosome cell death complexes through an expansion microscopy method. Cell Mol Life Sci. 79(10): 531.ArticlePubMedPMCPDF
- Xu YQ, Lv W, Wu HJ, Shi SF. 2020. Ginsenoside regulates Treg/Th17 cell ratio and inhibits inflammation to treat COPD. Die Pharmazie. 75(11): 590–594.ArticlePubMed
- Yang H, Oh KH, Kim HJ, Cho YH, Yoo YC. 2018. Ginsenoside-Rb2 and 20 (S)-Ginsenoside-Rg3 from Korean red ginseng prevent rotavirus infection in newborn mice. J Microbiol Biotechnol. 28(3): 391–396.ArticlePubMed
- Yoo DG, Kim MC, Park MK, Park KM, Quan FS, et al. 2012a. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS One. 7(3): e33678.ArticlePubMedPMC
- Yoo DG, Kim MC, Park MK, Song JM, Quan FS, et al. 2012b. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 15(10): 855–862.ArticlePubMedPMC
- Yoon J, Park B, Kim H, Choi S, Jung D. 2023. Korean red ginseng potentially improves maintaining antibodies after COVID-19 vaccination: A 24-week longitudinal study. Nutrients. 15(7): 1584.ArticlePubMedPMC
- Yu G, Choi YK, Lee S. 2024. Inflammasome diversity: exploring novel frontiers in the innate immune response. Trends Immunol. 45(4): 248–258.ArticlePubMed
- Yue PYK, Wong DYL, Wu PK, Leung PY, Mak NK, et al. 2006. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem Pharmacol. 72(4): 437–445.ArticlePubMed
- Yun TK. 2001. Panax ginseng--a non-organ-specific cancer preventive? Lancet Oncol. 2(1): 49–55.ArticlePubMed
- Yun TK. 2003. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res-Fund Mol M. 523-524: 63–74.ArticlePubMed
- Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, et al. 2020. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell. 180(6): 1115–1129.Article
- Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, et al. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 109(14): 5322–5327.ArticlePubMedPMC
- Zhao L, Zhang T, Zhang K. 2024. Pharmacological effects of ginseng and ginsenosides on intestinal inflammation and the immune system. Front Immunol. 15: 1353614.ArticlePubMedPMC
- Zheng M, Williams EP, Malireddi RS, Karki R, Banoth B, et al. 2020. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. 295(41): 14040–14052.ArticlePubMedPMC
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 90(3): 405–413.ArticlePubMed
Citations
Citations to this article as recorded by

- Pattern recognition receptors and inflammasome: Now and beyond
SuHyeon Oh, Young Ki Choi, SangJoon Lee
Molecules and Cells.2025; 48(8): 100239. CrossRef - Targeting innate immune sensors for therapeutic strategies in infectious diseases
Seyun Shin, Young Ki Choi, SangJoon Lee
Journal of Microbiology.2025; 63(6): e2503009. CrossRef - Formation and biological implications of Z-DNA
Yonghang Run, Mahmoud Tavakoli, Yuxuan Zhang, Karen M. Vasquez, Wenli Zhang
Trends in Genetics.2025;[Epub] CrossRef - mGem: Noncanonical nucleic acid structures—powerful but neglected antiviral targets
Václav Brázda, Richard P. Bowater, Petr Pečinka, Martin Bartas, Vinayaka R. Prasad
mBio.2025;[Epub] CrossRef - AIM2 drives inflammatory cell death and monkeypox pathogenesis
Jueun Oh, Yun-Ho Hwang, Jihye Lee, Cheong Seok, SuHyeon Oh, Hye Yoon Kim, Nabukenya Mariam, Jaeyoung Ahn, GyeongJu Yu, Jaewoo Park, Hayeon Kim, Suhyun Kim, Seyun Shin, Min-Chul Jung, Jinwoo Gil, Joo Sang Lee, Young Ki Choi, Dokeun Kim, Daesik Kim, You-Jin
Cellular & Molecular Immunology.2025; 22(12): 1615. CrossRef







 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article





