Articles
- Page Path
- HOME > J. Microbiol > Volume 63(2); 2025 > Article
-
Full article
Enoxacin adversely affects Salmonella enterica virulence and host pathogenesis through interference with type III secretion system type II (T3SS-II) and disruption of translocation of Salmonella Pathogenicity Island-2 (SPI2) effectors - El-Sayed Khafagy1, Gamal A. Soliman2,3, Maged S. Abdel-Kader4,5, Mahmoud M. Bendary6, Wael A. H. Hegazy7,8,*, Momen Askoura7
-
Journal of Microbiology 2025;63(2):e2410015.
DOI: https://doi.org/10.71150/jm.2410015
Published online: February 27, 2025
1Department of Pharmaceutics, College of Pharmacy, Prince Sattam bin Abdulaziz University, Al-kharj 11942, Saudi Arabia
2Department of Pharmacology, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
3Department of Pharmacology, College of Veterinary Medicine, Cairo University, Giza 12211, Egypt
4Department of Pharmacognosy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
5Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Alexandria 21215, Egypt
6Department of Microbiology and Immunology, Faculty of Pharmacy, Port Said University, Port Said 42511, Egypt
7Department of Microbiology and Immunology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt
8Department of Pharmaceutical Sciences, Pharmacy Program, Oman College of Health Sciences, Muscat 113, Oman
- *Correpondence Wael A. H. Hegazy waelmhegazy@daad-alumni.de
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,510 Views
- 61 Download
ABSTRACT
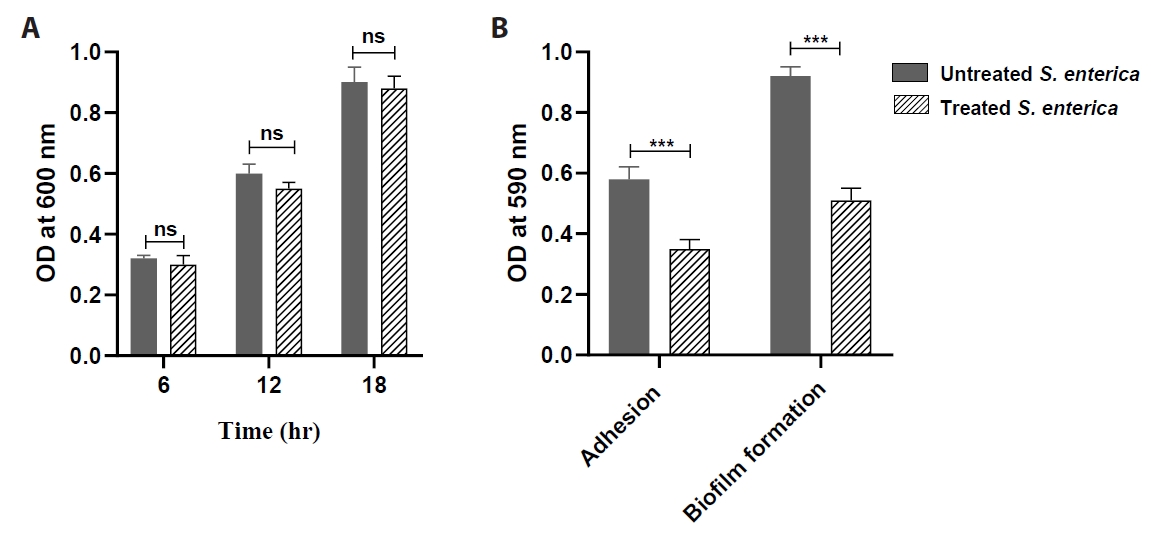
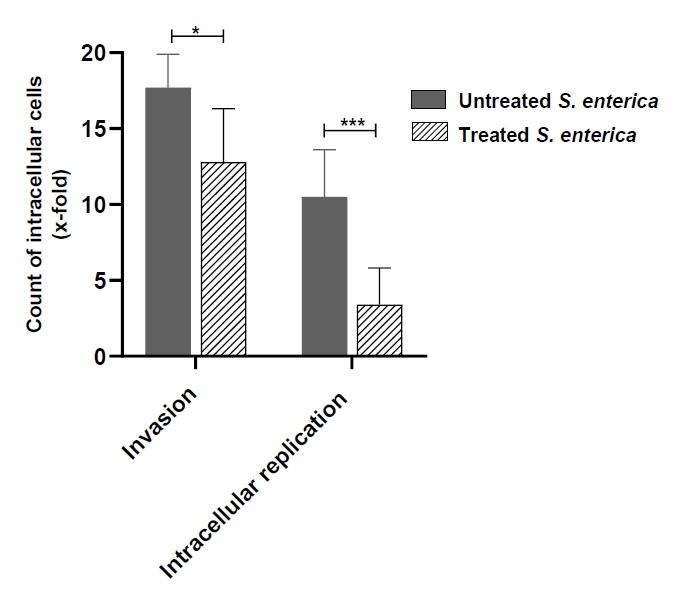
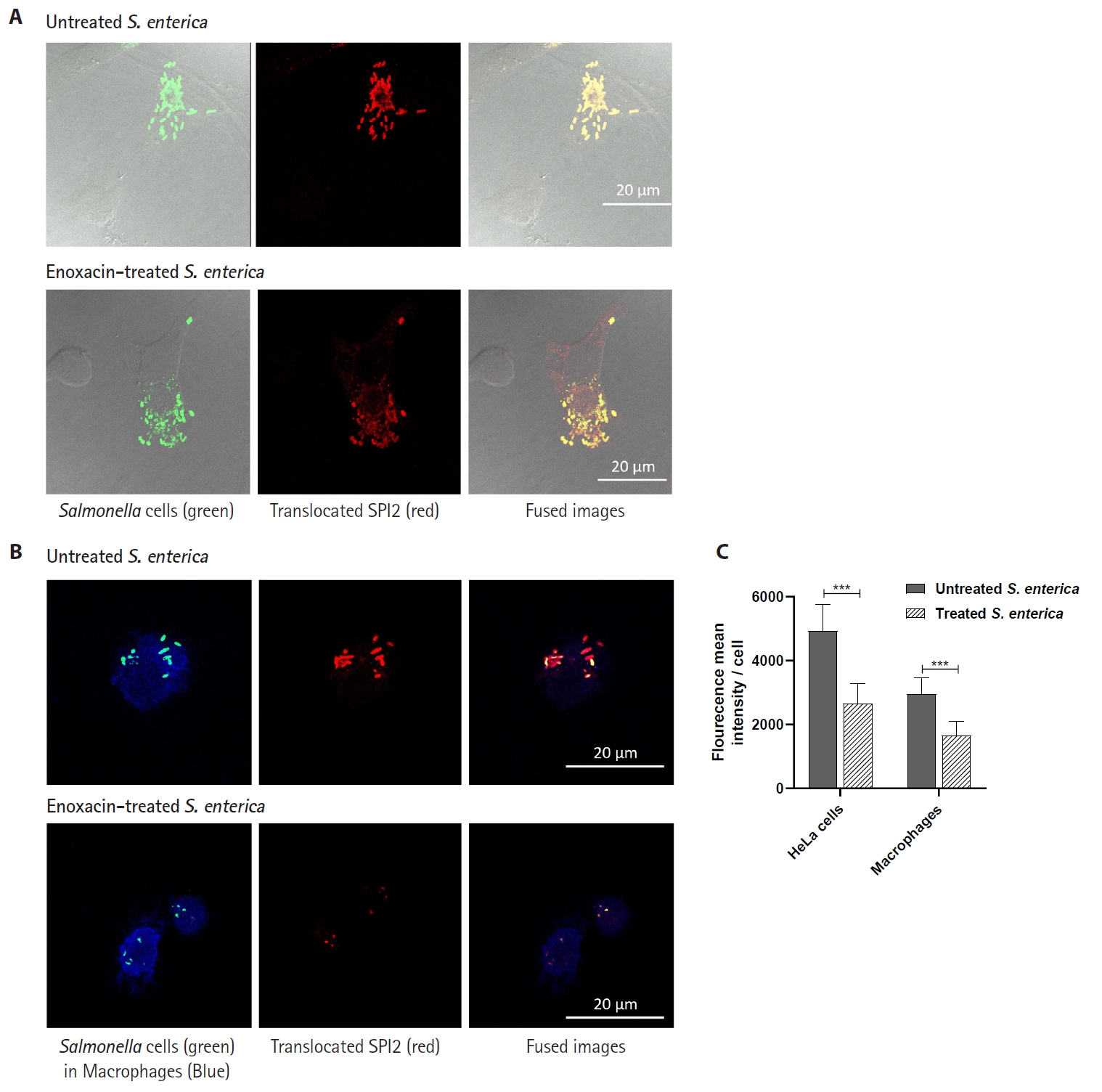
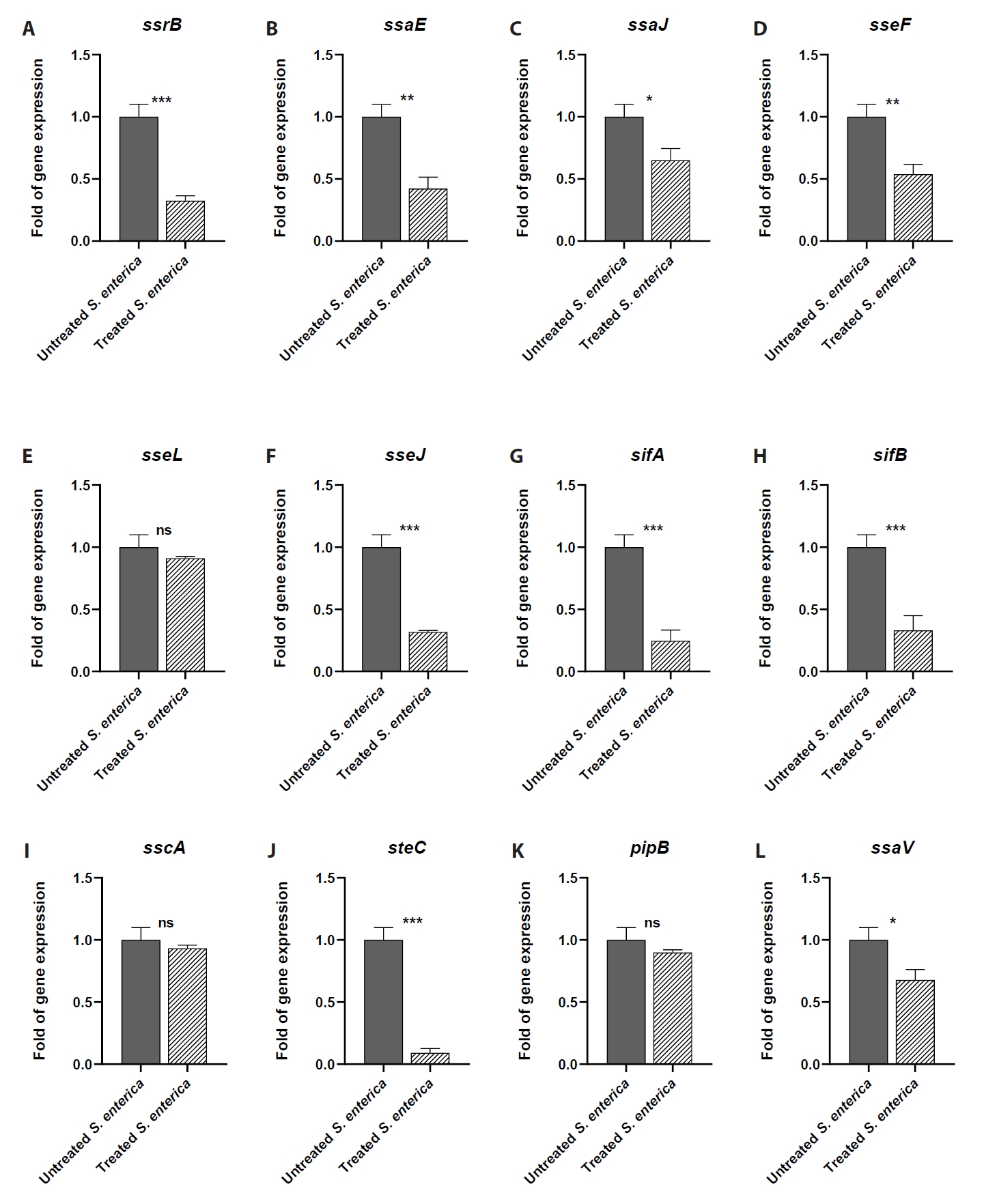
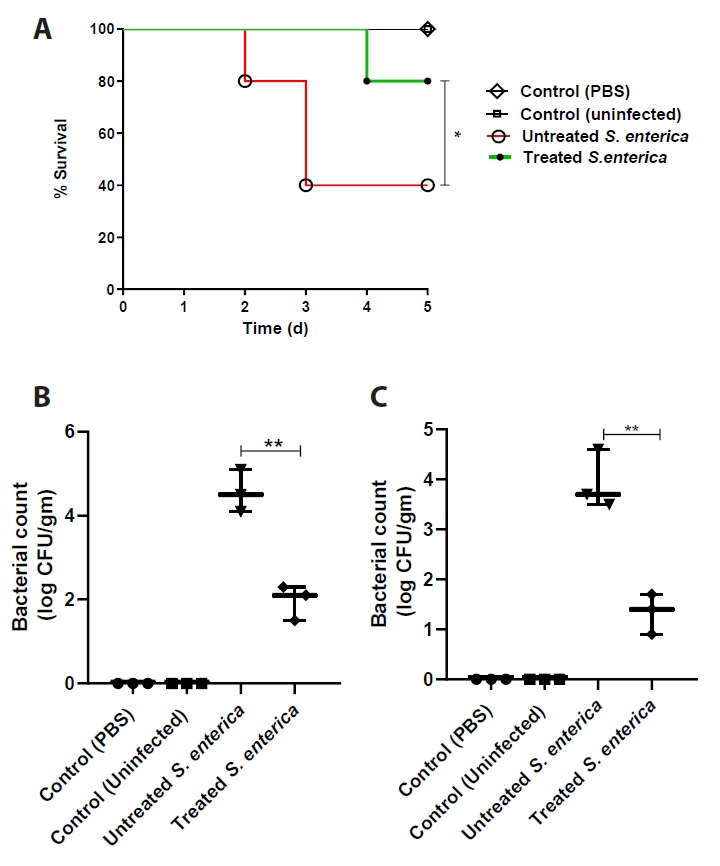
- Salmonella enterica is a clinically significant oro-fecal pathogen that causes a wide variety of illnesses and can lead to epidemics. S. enterica expresses a lot of virulence factors that enhance its pathogenesis in host. For instance, S. enterica employs a type three secretion system (T3SS) to translocate a wide array of effector proteins that could change the surrounding niche ensuring suitable conditions for the thrive of Salmonella infection. Many antimicrobials have been recently introduced to overcome the annoying bacterial resistance to antibiotics. Enoxacin is member of the second-generation quinolones that possesses a considerable activity against S. enterica. The present study aimed to evaluate the effect of enoxacin at sub-minimum inhibitory concentration (sub-MIC) on S. enterica virulence capability and pathogenesis in host. Enoxacin at sub-MIC significantly diminished both Salmonella invasion and intracellular replication within the host cells. The observed inhibitory effect of enoxacin on S. enterica internalization could be attributed to its ability to interfere with translocation of the T3SS effector proteins. These results were further confirmed by the finding that enoxacin at sub-MIC down-regulated the expression of the genes encoding for T3SS-type II (T3SS-II). Moreover, enoxacin at sub-MIC lessened bacterial adhesion to abiotic surface and biofilm formation which indicates a potential anti-virulence activity. Importantly, in vivo results showed a significant ability of enoxacin to protect mice against S. enterica infection and decreased bacterial colonization within animal tissues. In nutshell, current findings shed light on an additional mechanism of enoxacin at sub-MIC by interfering with Salmonella intracellular replication. The outcomes presented herein could be further invested in conquering bacterial resistance and open the door for additional effective clinical applications.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2024/03/29653).
Author Contributions
Conceptualization, M.A. and W. A. H. H.; methodology, El-S. K., G.A. S., M. S. A. and M. M. B. validation, El-S. K., G.A. S., M. S. A., M. M. B., M. A. and W. A. H.H.; formal analysis, El-S. K., G.A. S., M. S. A. and M. M. B.; investigation, El-S. K., G.A. S., M. S. A. and M. M. B.; resources, El-S. K., G.A. S., M. S. A., M. M. B., M. A. and W. A. H.H; data curation, El-S. K., G.A. S., M. S. A., M. M. B., M. A. and W. A. H. H. writing—original draft preparation, M. A. and W. A. H. H and W. A. H. H.; writing—review and editing, M. A. and W. A. H. H visualization, M. A. and W. A. H. H; supervision, W. A. H. H.; project administration, W. A. H. H.; funding acquisition, El-S. K. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Data Availability Statement
All the data are provided within this published article
Ethics Statement
The animal study was approved by the Faculty of Pharmacy, Port Said University Ethical Committee (Accession no. PSU.PHR.14). The study was conducted in accordance with the local legislation and institutional requirements.
- Abdulaal WH, Alhakamy NA, Asseri AH, Radwan MF, Ibrahim TS, et al. 2024. Redirecting pantoprazole as a metallo-beta-lactamase inhibitor in carbapenem-resistant Klebsiella pneumoniae. Front Pharmacol. 15: 1366459.ArticlePubMedPMC
- Agha KA, Abo-Dya NE, Ibrahim TS, Abdel-Aal EH, Hegazy WA. 2016. Benzotriazole-mediated synthesis and antibacterial activity of novel N-acylcephalexins. Sci Pharm. 84(3): 484–496. ArticlePubMedPMC
- Alandiyjany MN, Abdelaziz AS, Abdelfattah-Hassan A, Hegazy WAH, Hassan AA, et al. 2022. Novel in vivo assessment of antimicrobial efficacy of ciprofloxacin-loaded mesoporous silica nanoparticles against Salmonella typhimurium infection. Pharmaceuticals. 15(3): 357.ArticlePubMedPMC
- Alkhalil SS. 2024. Fluoroquinolone and enoxacin molecules are potential urease inhibitors for treating ureolytic bacterial infections. Mater Express. 14(6): 558–571. Article
- Almalki AJ, Ibrahim TS, Elhady SS, Darwish KM, Hegazy WAH. 2022. Repurposing α-adrenoreceptor blockers as promising anti-virulence agents in gram-negative bacteria. Antibiotics. 11(2): 178.ArticlePubMedPMC
- Alotaibi HF, Alotaibi H, Darwish KM, Khafagy ES, Abu Lila AS, et al. 2023. The anti-virulence activities of the antihypertensive drug propranolol in light of its anti-quorum sensing effects against Pseudomonas aeruginosa and Serratia marcescens. Biomedicines. 11(11): 3161.ArticlePubMedPMC
- Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 12(7): 465–478. ArticlePubMedPDF
- Askoura M, Almalki AJ, Lila ASA, Almansour K, Alshammari F, et al. 2021. Alteration of Salmonella enterica virulence and host pathogenesis through targeting SdiA by using the CRISPR-Cas9 system. Microorganisms. 9(12): 2564.ArticlePubMedPMC
- Askoura M, Hegazy WAH. 2020. Ciprofloxacin interferes with Salmonella typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog Dis. 78(8): ftaa051.ArticlePubMedPDF
- Badr-Eldin SM, Aldawsari HM, Ahmed OA, Kotta S, Abualsunun W, et al. 2024. Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa. Nanotechnol Rev. 13(1): 20230212.Article
- Bendary MM, Ali MA, Abdel Halim AS, Boufahja F, Chaudhary AA, et al. 2024. Investigating sulforaphane’s anti-virulence and anti-quorum sensing properties against Pseudomonas aeruginosa. Front Pharmacol. 15: 1406653.ArticlePubMedPMC
- Buckle GC, Walker CL, Black RE. 2012. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2(1): 010401.ArticlePubMedPMC
- Cavalu S, Elbaramawi SS, Eissa AG, Radwan MF, Ibrahim TS, et al. 2022. Characterization of the anti-biofilm and anti-quorum sensing activities of the β-adrenoreceptor antagonist atenolol against gram-negative bacterial pathogens. Int J Mol Sci. 23(21): 13088.ArticlePubMedPMC
- Charles PG. 2017. Enoxacin. In Kucers' the Use of Antibiotics, pp. 2171–2179, CRC Press.
- Cornelis GR. 2006. The type III secretion injectisome. Nat Rev Microbiol. 4(11): 811–825. ArticlePubMedPDF
- Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 28(4): 901–937. ArticlePubMedPMCPDF
- Deiwick J, Nikolaus T, Erdogan S, Hensel M. 1999. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 31(6): 1759–1773. ArticlePubMed
- Easmon CS, Crane JP, Blowers A. 1986. Effect of ciprofloxacin on intracellular organisms: In-vitro and in-vivo studies. J Antimicrob Chemother. 18 Suppl D: 43–48. ArticlePubMed
- Elfaky MA, Elbaramawi SS, Eissa AG, Ibrahim TS, Khafagy ES, et al. 2023. Drug repositioning: Doxazosin attenuates the virulence factors and biofilm formation in gram-negative bacteria. Appl Microbiol Biotechnol. 107(8): 3763–3778. ArticlePubMedPDF
- Elfaky MA, Okairy HM, Abdallah HM, Koshak AE, Mohamed GA, et al. 2024. Assessing the antibacterial potential of 6-gingerol: Combined experimental and computational approaches. Saudi Pharm J. 32(1): 102041.ArticlePubMedPMC
- Elfaky MA, Thabit AK, Eljaaly K, Zawawi A, Abdelkhalek AS, et al. 2022. Controlling of bacterial virulence: Evaluation of anti-virulence activities of prazosin against Salmonella enterica. Antibiotics (Basel). 11: Article
- Gerlach RG, Hensel M. 2007. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 120: 317–327. PubMed
- Gutiérrez-Castrellón P, Díaz-García L, de Colsa-Ranero A, Cuevas-Alpuche J, Jiménez-Escobar I. 2015. Efficacy and safety of ciprofloxacin treatment in urinary tract infections (UTIs) in adults: A systematic review with meta-analysis. Gac Med Mex. 151: 225–244. PubMed
- Harish BN, Menezes GA. 2011. Antimicrobial resistance in typhoidal salmonellae. Indian J. Med. Microbiol. 29(3): 223–229. ArticlePubMed
- Hegazy WA, Hensel M. 2012. Salmonella enterica as a vaccine carrier. Future Microbiol. 7(1): 111–127. ArticlePubMed
- Hegazy WA, Xu X, Metelitsa L, Hensel M. 2012. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect Immun. 80(4): 1193–1202. ArticlePubMedPMCPDF
- Hegazy WAH. 2015. Hepatitis C virus pathogenesis: Serum IL-33 level indicates liver damage. Afr. J. Microbiol. Res. 9(20): 1386–1393. Article
- Hegazy WAH, Abbas HA. 2017. Evaluation of the role of SsaV ‘Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr J Biotechnol. 16(13): 718–726. Article
- Hegazy WAH, Salem IM, Alotaibi HF, Khafagy ES, Ibrahim D. 2022. Terazosin interferes with quorum sensing and type III secretion system and diminishes the bacterial espionage to mitigate the Salmonella typhimurium pathogenesis. Antibiotics. 11(4): 465.ArticlePubMedPMC
- Heggie A, Cerny O, Holden DW. 2021. STEC and the intracellular Salmonella-induced F-actin meshwork. Cell Microbiol. 23(9): e13315. ArticlePubMedPDF
- Holzer SU, Hensel M. 2012. Divergent roles of Salmonella pathogenicity island 2 and metabolic traits during interaction of S. enterica serovar Typhimurium with host cells. PLoS One. 7(3): e33220. ArticlePubMedPMC
- Hung YT, Lay CJ, Wang CL, Koo M. 2017. Characteristics of nontyphoidal Salmonella gastroenteritis in Taiwanese children: A 9-year period retrospective medical record review. J Infect Public Health. 10(4): 518–521. ArticlePubMed
- Hussen NHA, Qadir SH, Rahman HS, Hamalaw YY, Kareem PSS, et al. 2023. Long-term toxicity of fluoroquinolones: A comprehensive review. Drug Chem Toxicol. 47(5): 795–806. ArticlePubMed
- Jałbrzykowska K, Chrzanowska A, Roszkowski P, Struga M. 2022. The new face of a well-known antibiotic: A review of the anticancer activity of enoxacin and its derivatives. Cancers. 14(12): 3056.ArticlePubMedPMC
- Kamaruzzaman NF, Kendall S, Good L. 2017. Targeting the hard to reach: Challenges and novel strategies in the treatment of intracellular bacterial infections. Br J Pharmacol. 174(12): 2225–2236. ArticlePubMedPMCPDF
- Khayat MT, Abbas HA, Ibrahim TS, Elbaramawi SS, Khayyat AN, et al. 2023. Synergistic benefits: Exploring the anti-virulence effects of metformin/vildagliptin antidiabetic combination against Pseudomonas aeruginosa via controlling quorum sensing systems. Biomedicines. 11(5): 1442.ArticlePubMedPMC
- Khayat MT, Elbaramawi SS, Nazeih SI, Safo MK, Khafagy ES, et al. 2023. Diminishing the pathogenesis of the food-borne pathogen Serratia marcescens by low doses of sodium citrate. Biology. 12(4): 504.ArticlePubMedPMC
- Khayat MT, Ibrahim TS, Darwish KM, Khayyat AN, Alharbi M, et al. 2022. Hiring of the anti-quorum sensing activities of hypoglycemic agent linagliptin to alleviate the Pseudomonas aeruginosa pathogenesis. Microorganisms. 10(8): 1541.ArticlePubMedPMC
- Khayat MT, Ibrahim TS, Khayyat AN, Alharbi M, Shaldam MA, et al. 2022. Sodium citrate alleviates virulence in Pseudomonas aeruginosa. Microorganisms. 10(5): 1046.ArticlePubMedPMC
- Knodler LA, Celli J, Hardt WD, Vallance BA, Yip C, et al. 2002. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol Microbiol. 43(5): 1089–1103. ArticlePubMed
- Knodler LA, Vallance BA, Hensel M, Jäckel D, Finlay BB, et al. 2003. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol Microbiol. 49(3): 685–704. ArticlePubMed
- Knuff K, Finlay BB. 2017. What the SIF is happening? The role of intracellular Salmonella-induced filaments. Front Cell Infect Microbiol. 7: 335.ArticlePubMedPMC
- Koshak AE, Okairy HM, Elfaky MA, Abdallah HM, Mohamed GA, et al. 2024. Antimicrobial and anti-virulence activities of 4-shogaol from grains of paradise against gram-negative bacteria: Integration of experimental and computational methods. J Ethnopharmacol. 323: 117611.ArticlePubMed
- Kuhle V, Hensel M. 2004. Cellular microbiology of intracellular Salmonella enterica: Functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell Mol Life Sci. 61(23): 2812–2826. ArticlePubMedPMCPDF
- Kwak YG, Choi SH, Kim T, Park SY, Seo SH, et al. 2017. Clinical guidelines for the antibiotic treatment for community-acquired skin and soft tissue infection. Infect Chemother. 49(4): 301–309. ArticlePubMedPMCPDF
- Lila ASA, Rajab AA, Abdallah MH, Rizvi SMD, Moin A, et al. 2023. Biofilm lifestyle in recurrent urinary tract infections. Life. 13(1): 148.ArticlePubMedPMC
- Linares JF, Gustafsson I, Baquero F, Martinez J. 2006. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci USA. 103(51): 19484–19489. ArticlePubMedPMC
- Liu X, Qu X, Wu C, Zhai Z, Tian B, et al. 2014. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials. 35(19): 5721–5730. ArticlePubMed
- Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. 2000. Salmonella pathogenicity islands: Big virulence in small packages. Microbes Infect. 2(2): 145–156. ArticlePubMed
- Molina-Quiroz R, Silva C, Molina C, Leiva L, Reyes-Cerpa S, et al. 2015. Exposure to sub-inhibitory concentrations of cefotaxime enhances the systemic colonization of Salmonella typhimurium in BALB/c mice. Open Biol. 5(7): Pii: 150070.ArticlePubMedPMCPDF
- Nazeih SI, Ali MA, Halim ASA, Al-Lawati H, Abbas HA, et al. 2023. Relocating glyceryl trinitrate as an anti-virulence agent against Pseudomonas aeruginosa and Serratia marcescens: Insights from molecular and in vivo investigations. Microorganisms. 11(7): 2420.ArticlePubMedPMC
- Patel JC, Galan JE. 2005. Manipulation of the host actin cytoskeleton by Salmonella—all in the name of entry. Curr Opin Microbiol. 8(1): 10–15. ArticlePubMed
- Poh J, Odendall C, Spanos A, Boyle C, Liu M, et al. 2008. SteC is a Salmonella kinase required for SPI-2-dependent F-actin remodelling. Cell Microbiol. 10(1): 20–30. ArticlePubMedPMC
- Rahman BA, Wasfy MO, Maksoud MA, Hanna N, Dueger E, et al. 2014. Multi-drug resistance and reduced susceptibility to ciprofloxacin among Salmonella enterica serovar Typhi isolates from the Middle East and Central Asia. New Microbes New Infect. 2(2): 88–92. ArticlePubMedPMC
- Rajab AA, Hegazy WA. 2023. What’s old is new again: Insights into diabetic foot microbiome. World J Diabetes. 14(12): 680–704. ArticlePubMedPMC
- Redgrave LS, Sutton SB, Webber MA, Piddock LJV. 2014. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 22(8): 438–445.ArticlePubMed
- Schmidt H, Hensel M. 2004. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 17(1): 14–56. ArticlePubMedPMCPDF
- Shaw A, Gullerova M. 2021. Home and away: The role of non-coding RNA in intracellular and intercellular DNA damage response. Genes. 12(9): 1475.ArticlePubMedPMC
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 40(2): 175–179. ArticlePubMed
- Tadesse G, Tessema TS, Beyene G, Aseffa A. 2018. Molecular epidemiology of fluoroquinolone resistant Salmonella in Africa: A systematic review and meta-analysis. PLoS One. 13(2): e0192575. ArticlePubMedPMC
- Thabit AK, Eljaaly K, Zawawi A, Ibrahim TS, Eissa AG, et al. 2022a. Muting bacterial communication: Evaluation of prazosin anti-quorum sensing activities against gram-negative bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens. Biology (Basel). 11(5): Article
- Thabit AK, Eljaaly K, Zawawi A, Ibrahim TS, Eissa AG, et al. 2022b. Silencing of Salmonella typhimurium pathogenesis: Atenolol acquires efficient anti-virulence activities. Microorganisms. 10(10): 1976.ArticlePubMedPMC
- Troman LA, Collinson I. 2021. Pushing the envelope: The mysterious journey through the bacterial secretory machinery, and beyond. Front Microbiol. 12: 782900.ArticlePubMedPMC
- Uche IV, MacLennan CA, Saul A. 2017. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 11(3): e0005118. ArticlePubMedPMC
- Vernikos GS, Parkhill J. 2006. Interpolated variable order motifs for identification of horizontally acquired DNA: Revisiting the Salmonella pathogenicity islands. Bioinformatics. 22(18): 2196–2203. ArticlePubMedPDF
- Vesterlund S, Paltta J, Karp M, Ouwehand AC. 2005. Measurement of bacterial adhesion—In vitro evaluation of different methods. J Microbiol Methods. 60(2): 225–233. ArticlePubMed
- Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. 2015. Typhoid fever. Lancet. 385(9973): 1136–1145. ArticlePubMed
- Xu X, Hegazy WA, Guo L, Gao X, Courtney AN, et al. 2014. Effective cancer vaccine platform based on attenuated Salmonella and a type III secretion system. Cancer Res. 74(22): 6260–6270. ArticlePubMedPMCPDF
- Yao C, Zhu M, Han X, Xu Q, Dai M, et al. 2021. A bone-targeting enoxacin delivery system to eradicate Staphylococcus aureus-related implantation infections and bone loss. Front Bioeng Biotechnol. 9(1): 749910.ArticlePubMedPMC
- Yin J, Chen Y, Xie X, Xia J, Li Q, et al. 2017. Influence of Salmonella enterica serovar Pullorum pathogenicity island 2 on type III secretion system effector gene expression in chicken macrophage HD11 cells. Avian Pathol. 46(3): 209–214. ArticlePubMed
- Yu XJ, Grabe GJ, Liu M, Mota LJ, Holden DW. 2018. SsaV interacts with SsaL to control the translocon-to-effector switch in the Salmonella SPI-2 type three secretion system. MBio. 9(6): 10.1128/mbio.01149-18. ArticlePDF
- Zehra F, Fatima SM, Syedain F. 2015. Sensitivity pattern of Salmonella species in different age groups. Int J Endors Health Sci Res. 3(1): 34–37. Article
References
Figure & Data
References
Citations

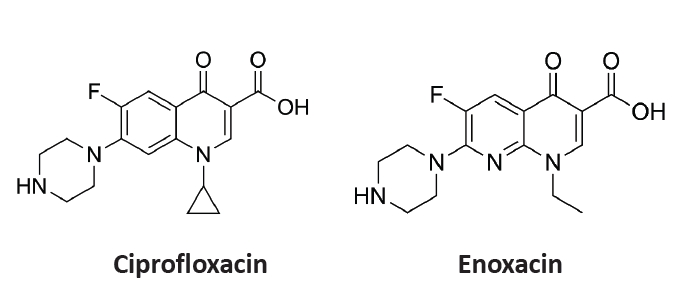
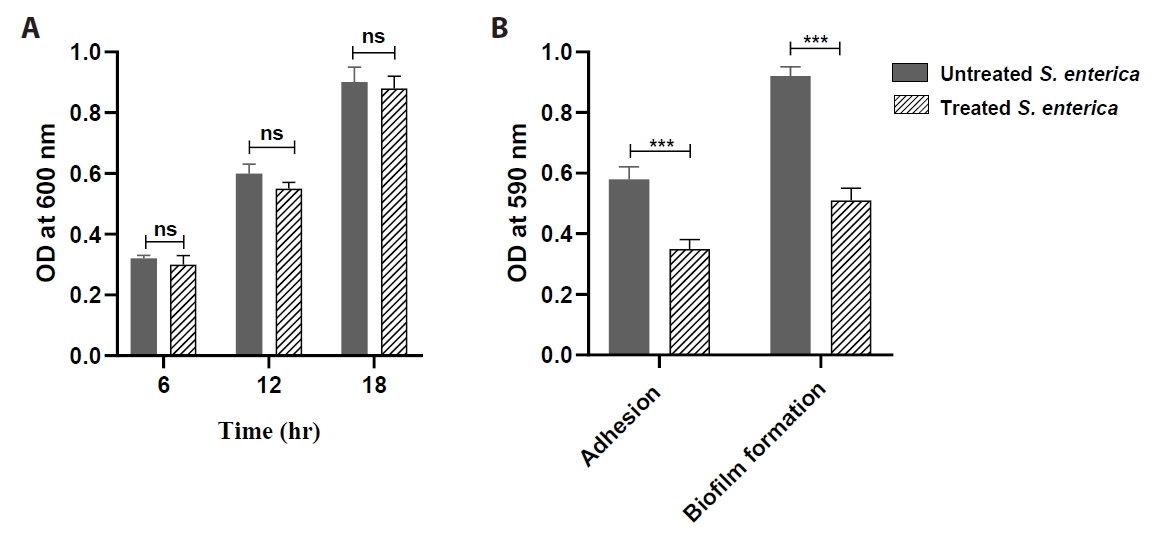
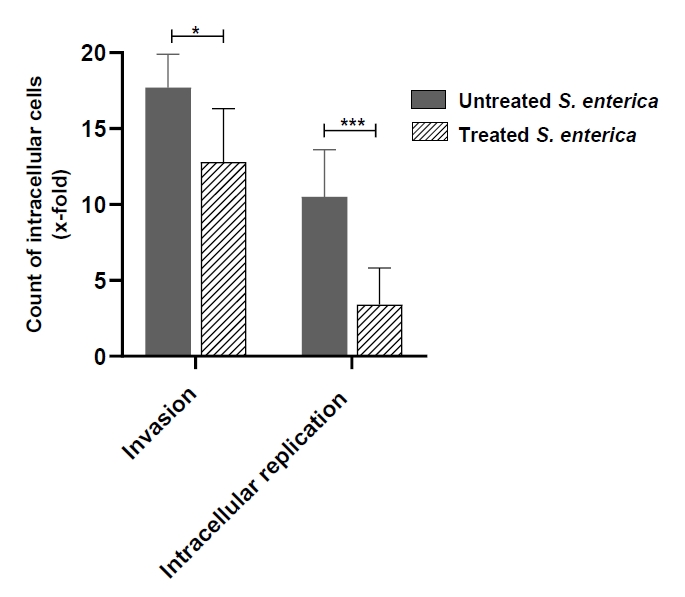
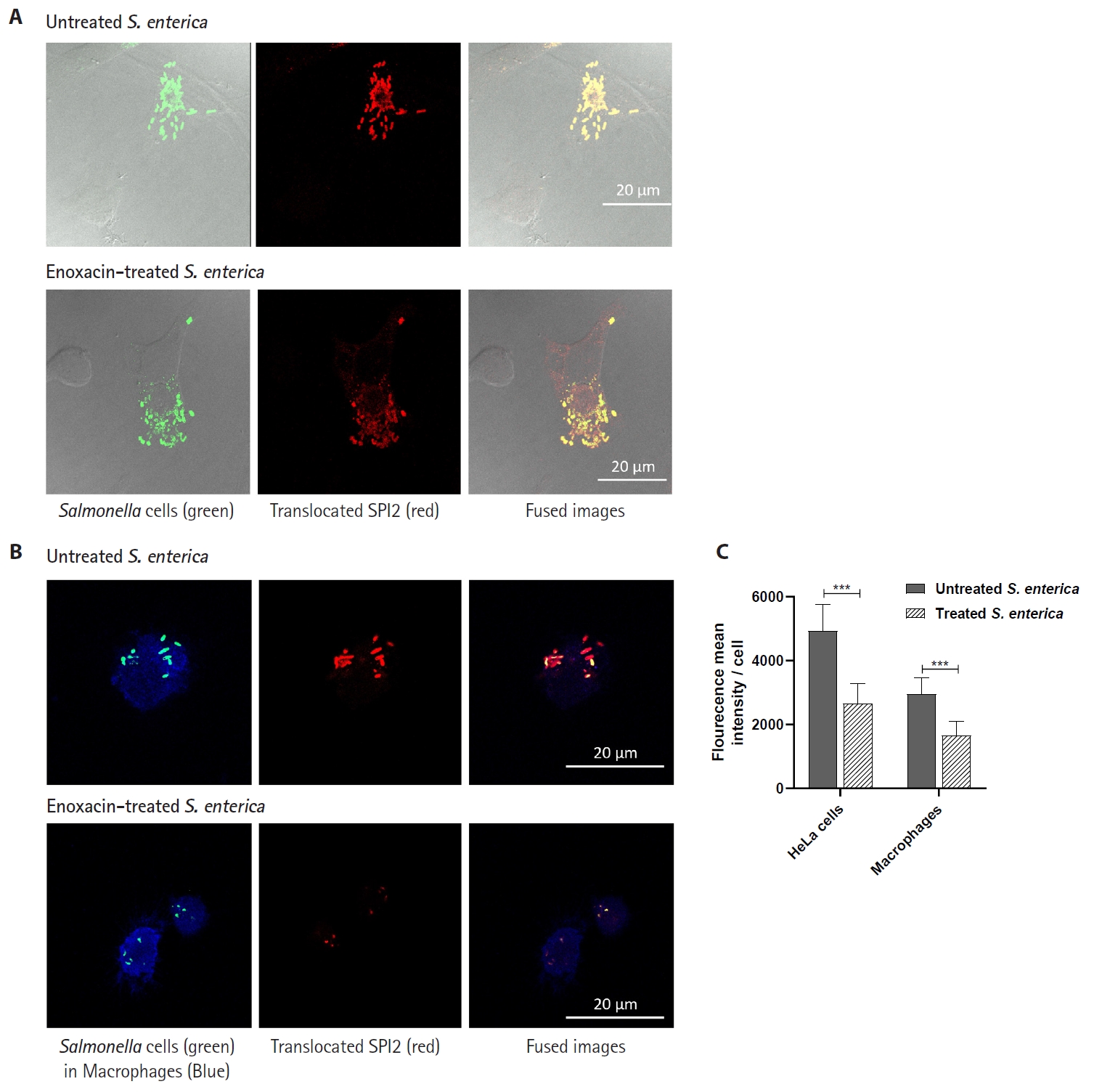
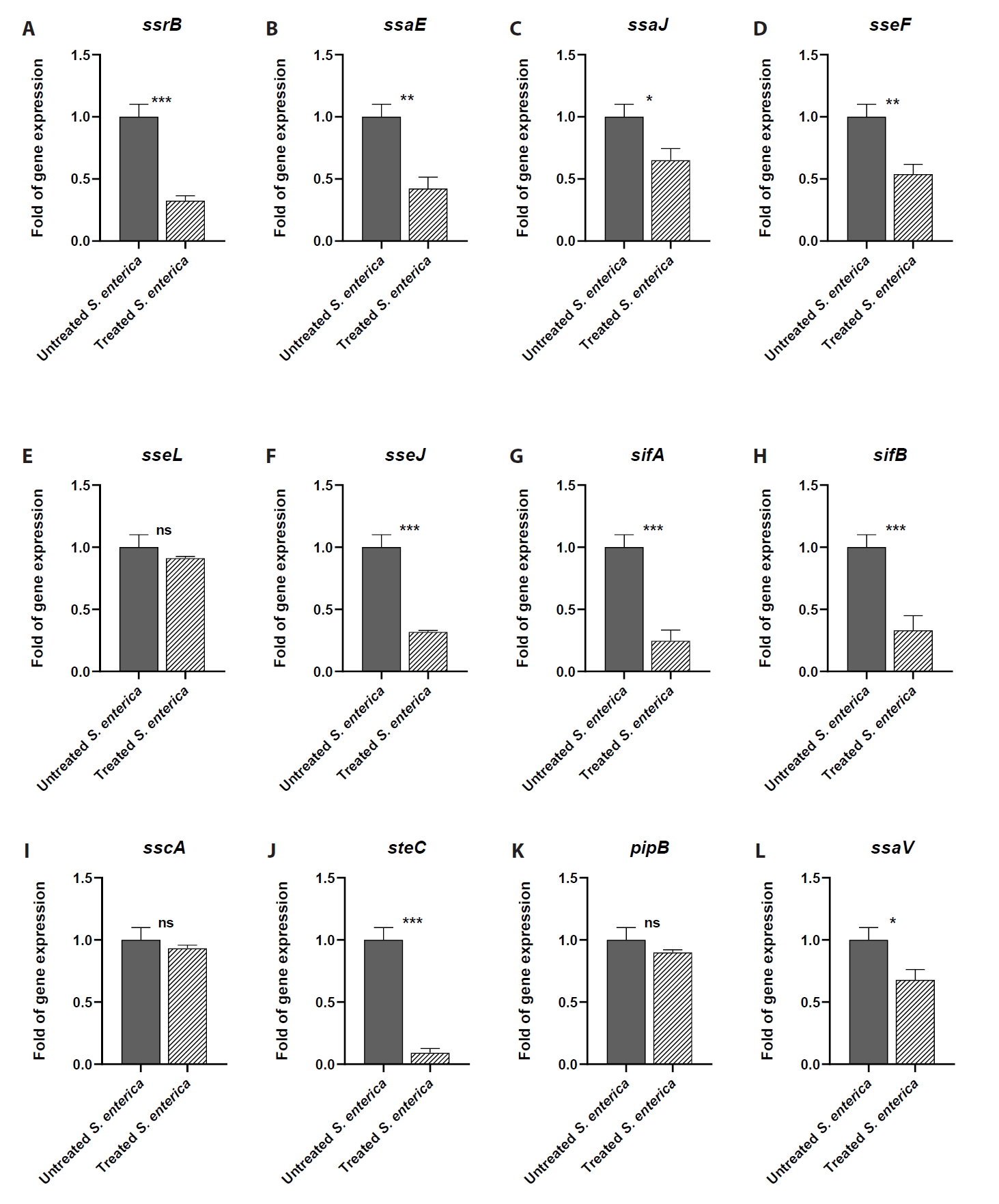
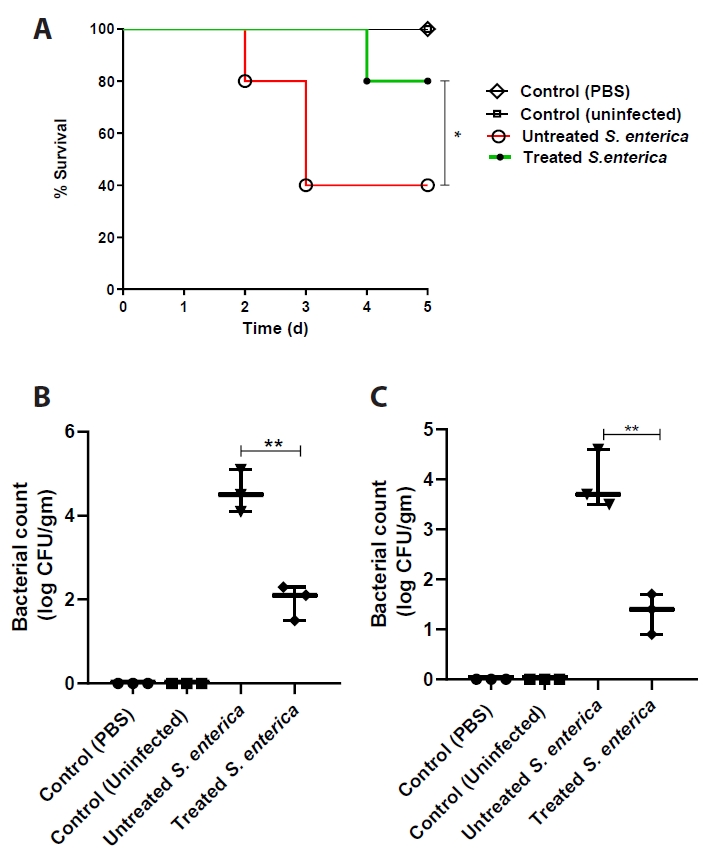
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article







