Articles
- Page Path
- HOME > J. Microbiol > Volume 63(5); 2025 > Article
-
Full article
Alizarin, which reduces ExoS, attenuates inflammation by P. aeruginosa in H292 cells - Seung-Ho Kim1,2, Hye In Ahn4, Jae-Hoon Oh5, Da Yun Seo3, Jung-Hee Kim1, Ok-kyoung Kwon1, Ji-Won Park6,7,*, Kyung-Seop Ahn1,*
-
Journal of Microbiology 2025;63(5):e2411012.
DOI: https://doi.org/10.71150/jm.2411012
Published online: May 27, 2025
1Natural Medicine Research Center, Korea Research Institute of Bioscience and Biotechnology, Chungbuk 28116, Republic of Korea
2College of Life Sciences and Biotechnology, Korea University, Seoul, 02841, Republic of Korea
3ELEO Inc., Daejeon 34141, Republic of Korea
4Stem Cell Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 34141, Republic of Korea
5AptaBio, Gyeonggi-do 16954, Republic of Korea
6Division of Practical Research, Honam National Institute of Biologucal Resources (HNIBR), Jeollanam-do 58762, Republic of Korea
7Advanced Research Center for Island Wildlife Biomaterials, Honal Natinal Institute of Biological Resources, Jeollanam-do 58762, Republic of Korea
- *Correspondence Ji-Won Park jjiwon87@gmail.com, jjiwon2@hnibr.re.kr Kyung-Seop Ahn ksahn@kribb.re.kr
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
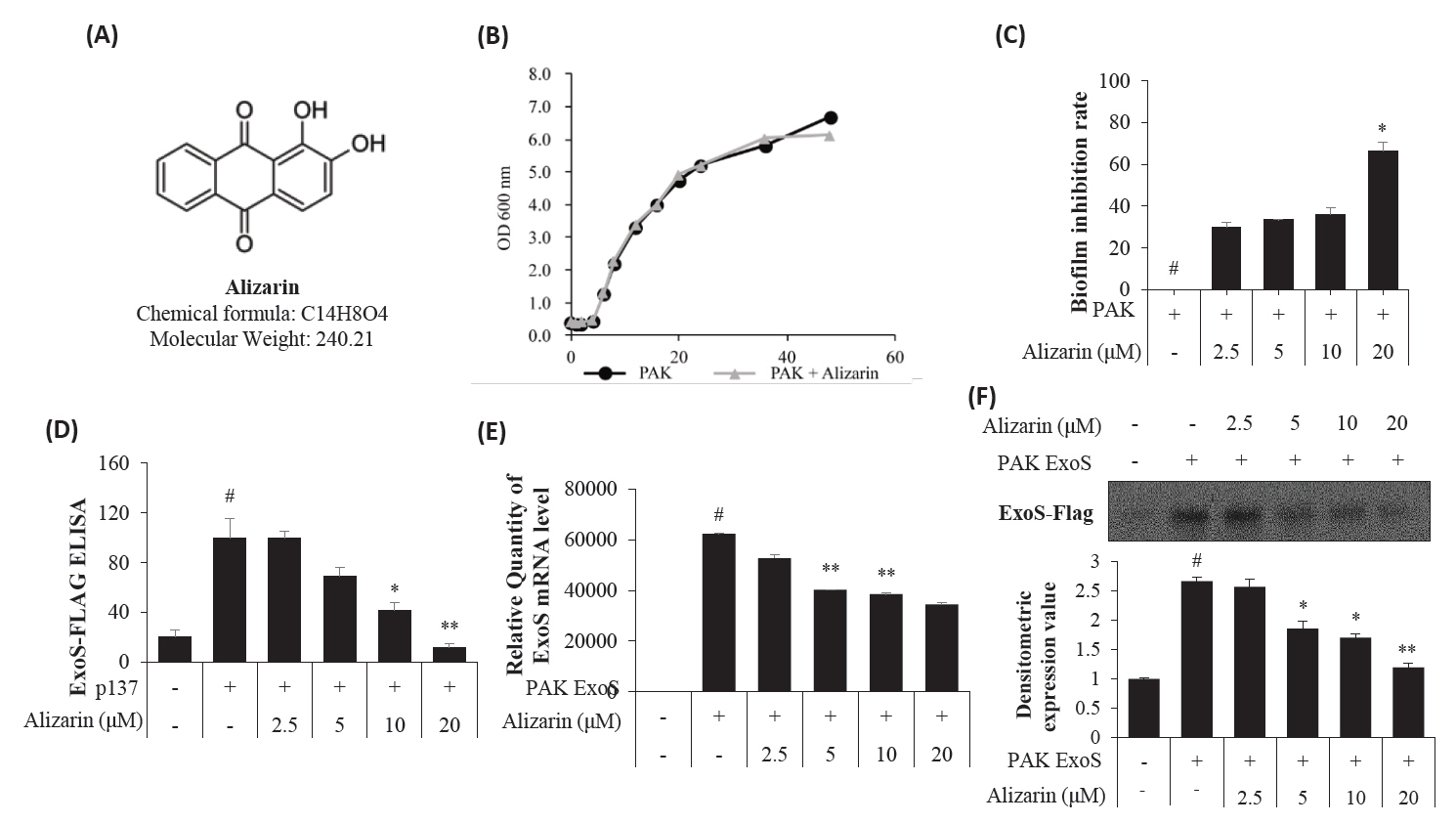
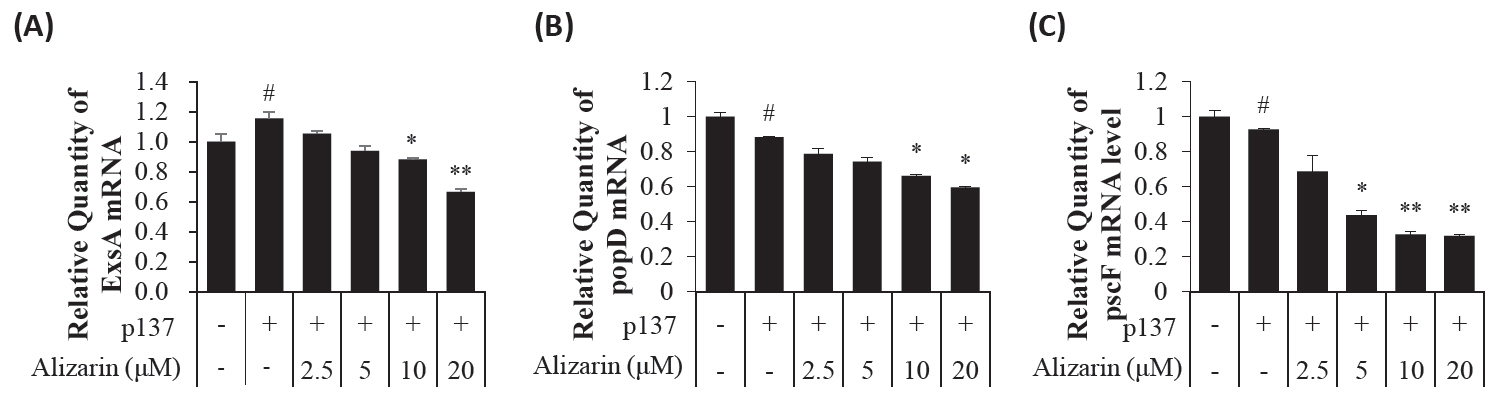
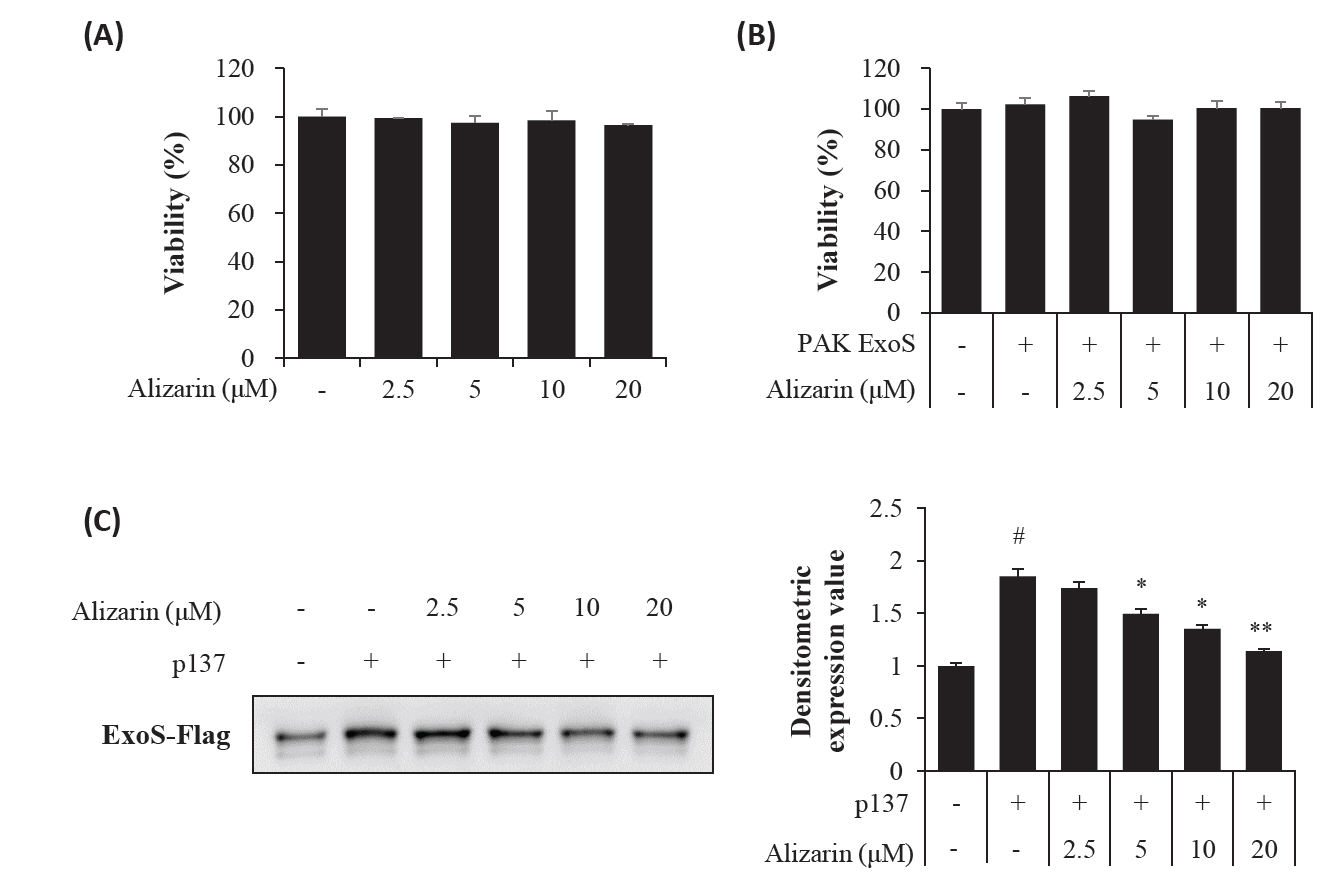
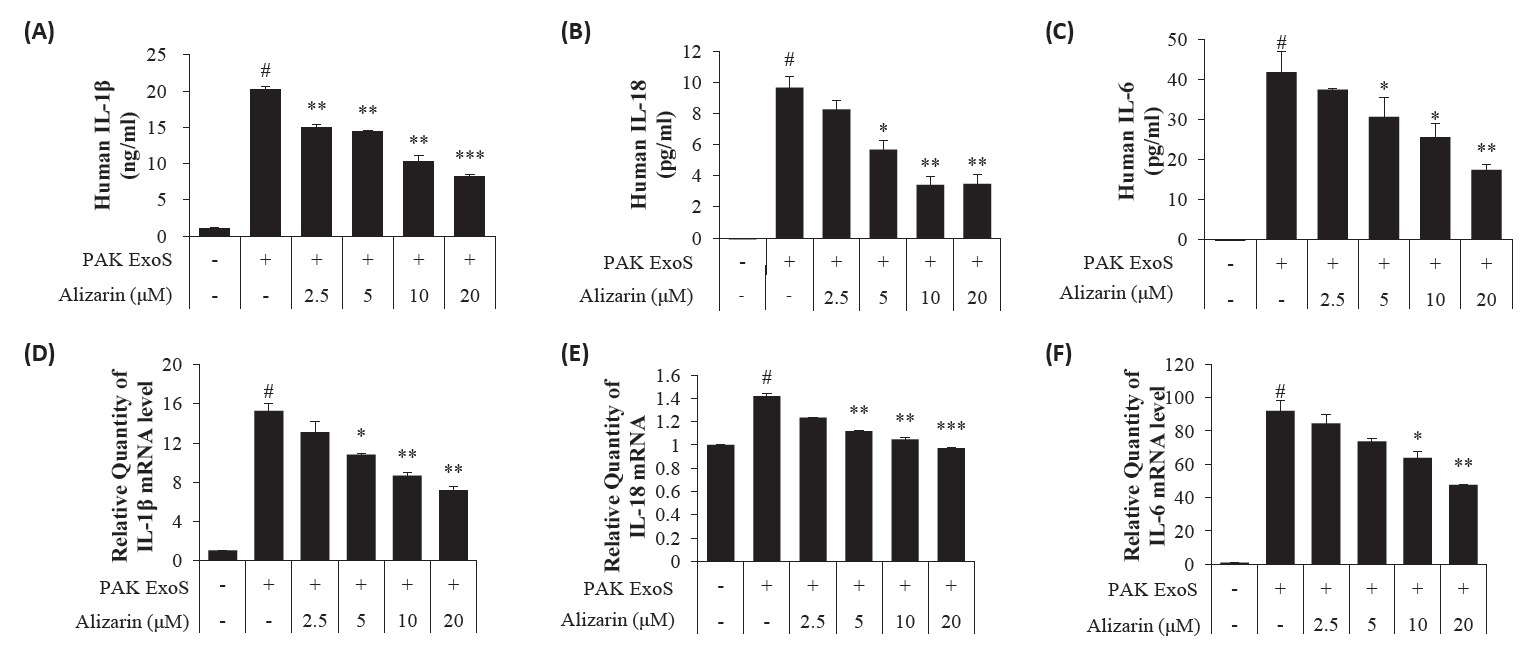
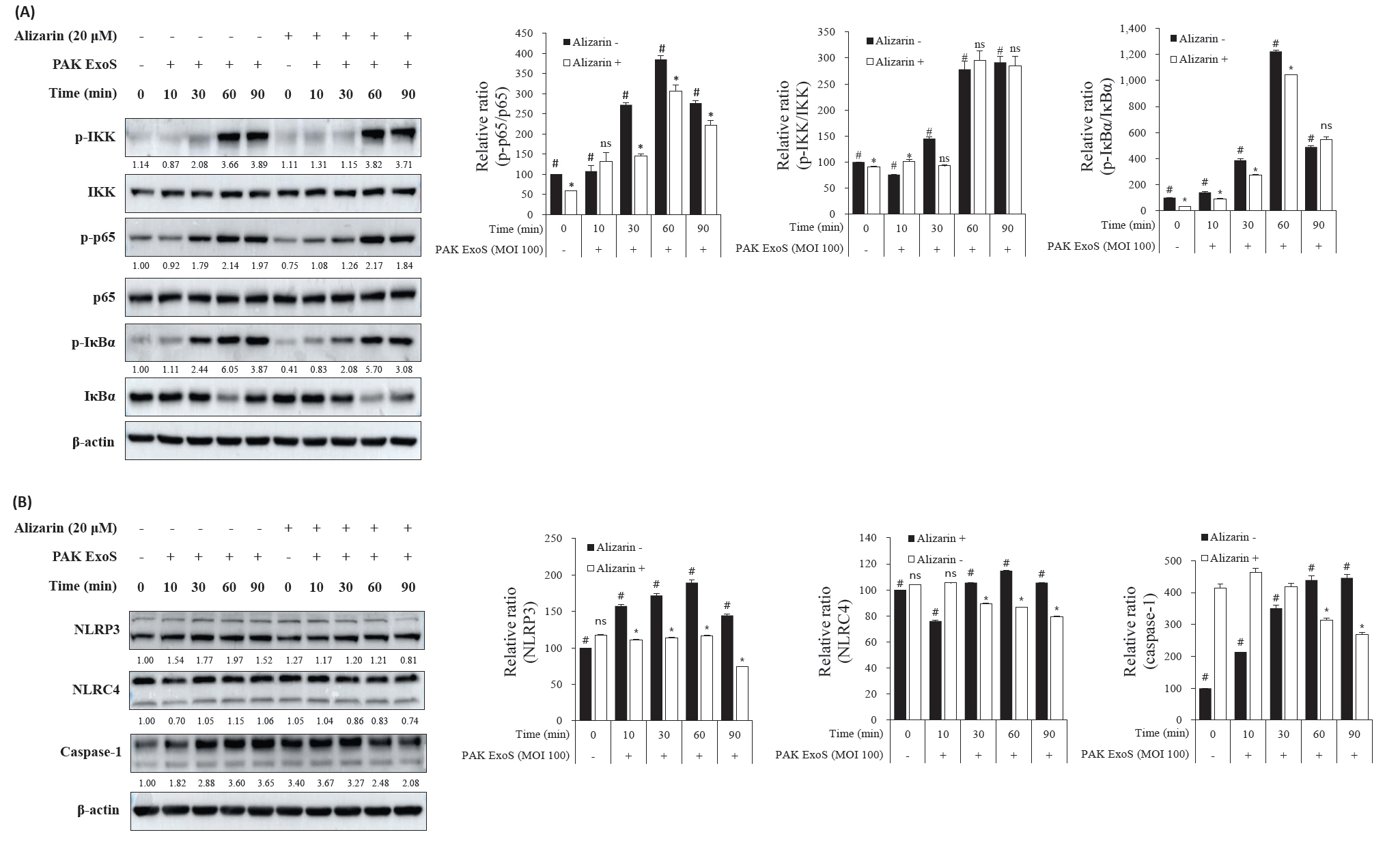
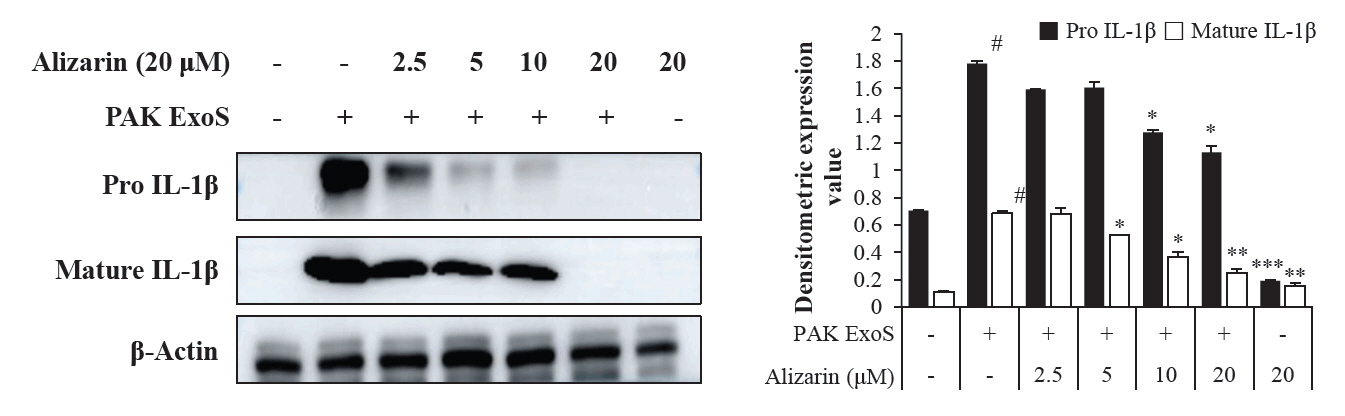
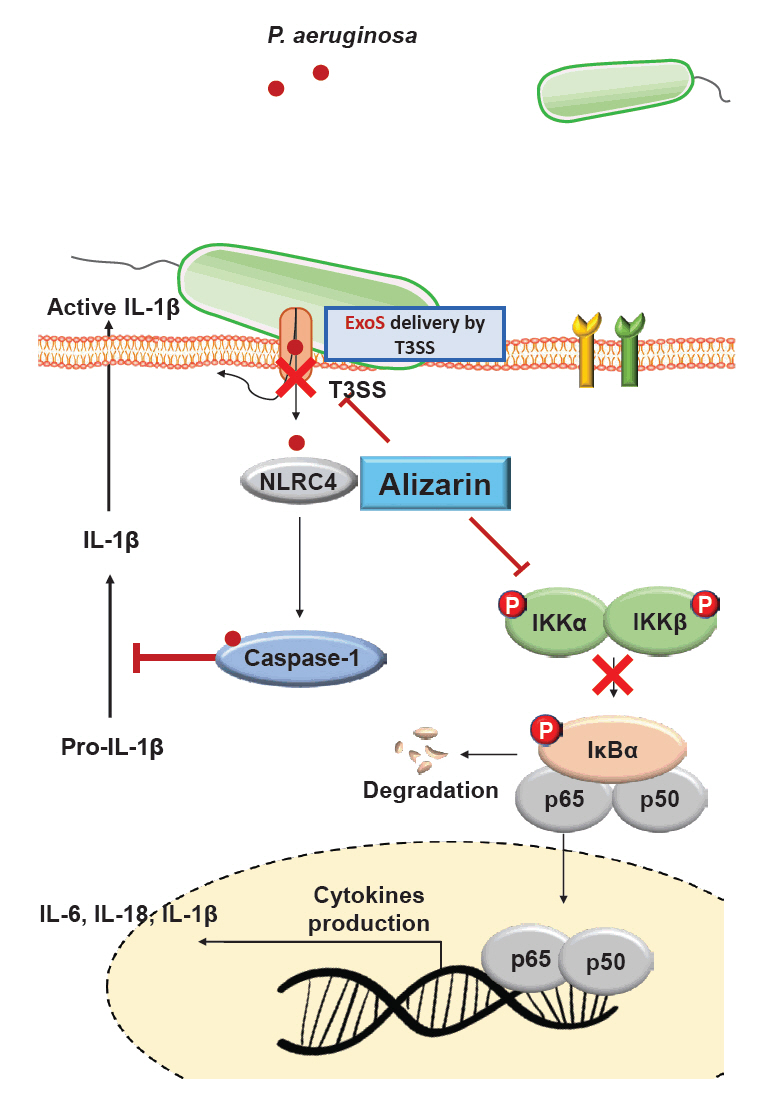
- Pseudomonas aeruginosa (P. aeruginosa) is resistant to several drugs as well as antibiotics and is thus classified as multidrug resistant and extensively drug resistant. These bacteria have a secretion system called the "type 3 secretion system (T3SS)", which facilitates infection by delivering an effector protein. ExoenzymeS (ExoS) is known to induce cell death and activate caspase-1. In particular, patients infected with P. aeruginosa develop diseases associated with high mortality, such as pneumonia, because no drug targets an ExoS or T3SS. We selected natural compounds to treat T3SS-mediated pneumonia and chose alizarin, a red dye. We confirmed the effects of alizarin on T3SS by bacterial PCR and ELISA. It was confirmed that alizarin regulates ExoS by inhibiting exsA but also popD and pscF. Furthermore, in infected H292 cells, it not only attenuates inflammation by inhibiting lipopolysaccharide (LPS)-induced phosphorylation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 but also interferes with the level of ExoS delivered into the host and modulates caspase-1. We confirmed this result and determined that it led to decreases in proinflammatory cytokines such as Interleukin-1beta (IL-1β), Interleukin-6 (IL-6), and Interleukin-18 (IL-18). Therefore, we suggest that alizarin is a suitable drug for treating pneumonia caused by P. aeruginosa because it helps to attenuate inflammation by regulating T3SS and NF-κB signaling.
Introduction
Materials and Methods
Results
Discussion
Conclusion
Acknowledgments
This study was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (grant number: NRF-2020R1A2C2101228) and by the Honam National Institute of Biological Resources (HNIBR202302113), funded by the Korea Ministry of Environment (MOE). And awarded to the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM1202511) of the Republic of Korea. The funders had no role in data analysis, study design or preparation of the manuscript.
Authors Contribution
SHK carried out all experiments and prepared the manuscript. HIA mainly contributed to conducting real-time PCR and ELISA experiments. OKK and JHK contributed to the preparation of the experimental materials. JHO and DYS assisted with the in vitro experiments. KSA and JWP prepared and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no competing interests.
- Al Moussawi K, Kazmierczak BI. 2014. Distinct contributions of interleukin-1alpha (IL-1alpha) and IL-1beta to innate immune recognition of Pseudomonas aeruginosa in the lung. Infect Immun. 82: 4204–4211. ArticlePubMedPMCPDF
- Armentrout EI, Rietsch A. 2016. The Type III secretion translocation pore senses host cell contact. PLoS Pathog. 12: e1005530. ArticlePubMedPMC
- Azimi S, Kafil HS, Baghi HB, Shokrian S, Najaf K, et al. 2016. Presence of exoY, exoS, exoU and exoT genes, antibiotic resistance and biofilm production among Pseudomonas aeruginosa isolates in Northwest Iran. GMS Hyg Infect Control. 11: Doc04.ArticlePubMedPMC
- Burkinshaw BJ, Strynadka NC. 2014. Assembly and structure of the T3SS. Biochim Biophys Acta. 1843: 1649–1663. ArticlePubMed
- Cohen TS, Prince AS. 2013. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 123: 1630–1637. ArticlePubMedPMC
- Cornelis GR, Wolf-Watz H. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 23: 861–867. ArticlePubMedPDF
- Diaz MR, King JM, Yahr TL. 2011. Intrinsic and extrinsic regulation of Type III secretion gene expression in Pseudomonas aeruginosa. Front Microbiol. 2: 89.ArticlePubMedPMC
- Dongari-Bagtzoglou A. 2008. Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti Infect Ther. 6: 201–208. ArticlePubMedPMC
- Du Toit A. 2015. Biofilms: The T3SS translocon in biofilm formation. Nat Rev Microbiol. 13: 2–3. ArticlePDF
- Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 7: 654–665. ArticlePubMedPMCPDF
- Henriksson ML, Sundin C, Jansson AL, Forsberg A, Palmer RH, et al. 2002. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Biochem J. 367: 617–628. ArticlePubMedPMCPDF
- Kaminski A, Gupta KH, Goldufsky JW, Lee HW, Gupta V, et al. 2018. Pseudomonas aeruginosa ExoS induces intrinsic apoptosis in target host cells in a manner that is dependent on its GAP domain activity. Sci Rep. 8: 14047.ArticlePubMedPMCPDF
- Kang JS, Moon C, Mun SJ, Lee JE, Lee SO, et al. 2021. Antimicrobial susceptibility trends and risk factors for antimicrobial resistance in Pseudomonas aeruginosa bacteremia: 12-year experience in a tertiary hospital in Korea. J Korean Med Sci. 36: e273.ArticlePubMedPMCPDF
- Kim J, Ahn K, Min S, Jia J, Ha U, et al. 2005. Factors triggering Type III secretion in Pseudomonas aeruginosa. Microbiology (Reading). 151: 3575–3587. ArticlePubMed
- Luna RA, Millecker LA, Webb CR, Mason SK, Whaley EM, et al. 2013. Molecular epidemiological surveillance of multidrug-resistant Pseudomonas aeruginosa isolates in a pediatric population of patients with cystic fibrosis and determination of risk factors for infection with the Houston-1 strain. J Clin Microbiol. 51: 1237–1240. ArticlePubMedPMCPDF
- Marsden AE, King JM, Spies MA, Kim OK, Yahr TL. 2016. Inhibition of Pseudomonas aeruginosa ExsA DNA-binding activity by N-hydroxybenzimidazoles. Antimicrob Agents Chemother. 60: 766–776. ArticlePubMedPMCPDF
- Morales E, Cots F, Sala M, Comas M, Belvis F, et al. 2012. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv Res. 12: 122.ArticlePubMedPMCPDF
- Mori N, Oishi K, Sar B, Mukaida N, Nagatake T, et al. 1999. Essential role of transcription factor nuclear factor-kappaB in regulation of interleukin-8 gene expression by nitrite reductase from Pseudomonas aeruginosa in respiratory epithelial cells. Infect Immun. 67: 3872–3878. ArticlePubMedPMCPDF
- Moser C, Jensen PO, Thomsen K, Kolpen M, Rybtke M, et al. 2021. Immune responses to Pseudomonas aeruginosa biofilm infections. Front Immunol. 12: 625597.ArticlePubMedPMC
- Park WS, Lee J, Na G, Park S, Seo SK, et al. 2022. Benzyl isothiocyanate attenuates inflammasome activation in Pseudomonas aeruginosa LPS-stimulated THP-1 cells and exerts regulation through the MAPKs/NF-κB pathway. Int J Mol Sci. 23: 1228.ArticlePubMedPMC
- Pederson KJ, Vallis AJ, Aktories K, Frank DW, Barbieri JT. 1999. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 32: 393–401. ArticlePubMed
- Preciado D, Caicedo E, Jhanjee R, Silver R, Harris G, et al. 2005. Pseudomonas aeruginosa lipopolysaccharide induction of keratinocyte proliferation, NF-kappaB, and cyclin D1 is inhibited by indomethacin. J Immunol. 174: 2964–2973. ArticlePubMed
- Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 171: 1209–1223. ArticlePubMedPMC
- Shrestha M, Xiao Y, Robinson H, Schubot FD. 2015. Structural analysis of the regulatory domain of ExsA, a key transcriptional regulator of the type three secretion system in Pseudomonas aeruginosa. PLoS One. 10: e0136533. ArticlePubMedPMC
- Sun J, Barbieri JT. 2004. ExoS Rho GTPase-activating protein activity stimulates reorganization of the actin cytoskeleton through Rho GTPase guanine nucleotide disassociation inhibitor. J Biol Chem. 279: 42936–42944. ArticlePubMed
- Sun J, LaRock DL, Skowronski EA, Kimmey JM, Olson J, et al. 2020. The Pseudomonas aeruginosa protease LasB directly activates IL-1β. EBioMedicine. 60: 102984.ArticlePubMedPMC
- Ta A, Vanaja SK. 2021. Inflammasome activation and evasion by bacterial pathogens. Curr Opin Immunol. 68: 125–133. ArticlePubMed
- Tang Y, Romano FB, Breña M, Heuck AP. 2018. The Pseudomonas aeruginosa type III secretion translocator PopB assists the insertion of the PopD translocator into host cell membranes. J Biol Chem. 293: 8982–8993. ArticlePubMedPMC
- Tran CS, Rangel SM, Almblad H, Kierbel A, Givskov M, et al. 2014. The Pseudomonas aeruginosa type III translocon is required for biofilm formation at the epithelial barrier. PLoS Pathog. 10: e1004479.ArticlePubMedPMC
- Trovato V, Mezzi A, Brucale M, Rosace G, Plutino MR. 2022. Alizarin-functionalized organic-inorganic silane coatings for the development of wearable textile sensors. J Colloid Interface Sci. 617: 463–477. ArticlePubMed
- Tummler B. 2019. Emerging therapies against infections with Pseudomonas aeruginosa. F1000Res. 8: 1374.
- Ulland TK, Ferguson PJ, Sutterwala FS. 2015. Evasion of inflammasome activation by microbial pathogens. J Clin Invest. 125: 469–477. ArticlePubMedPMC
- Wagener BM, Anjum N, Christiaans SC, Banks ME, Parker JC, et al. 2020a. Exoenzyme Y contributes to end-organ dysfunction caused by Pseudomonas aeruginosa pneumonia in critically ill patients: an exploratory study. Toxins (Basel). 12: 369.Article
- Wagener BM, Anjum N, Evans C, Brandon A, Honavar J, et al. 2020b. Alpha-tocopherol attenuates the severity of Pseudomonas aeruginosa-induced pneumonia. Am J Respir Cell Mol Biol. 63: 234–243. Article
- Xu Z, Hou Y, Zou C, Liang H, Mu J, et al. 2022. Alizarin, a natural compound, inhibits the growth of pancreatic cancer cells by abrogating NF-kappaB activation. Int J Biol Sci. 18: 2759–2774. ArticlePubMedPMC
- Zhang J, Chi Y, Feng L. 2021. The mechanism of degradation of alizarin red by a white-rot fungus Trametes gibbosa. BMC Biotechnol. 21: 64.ArticlePubMedPMCPDF
- Zhang Y, Zhang C, Du X, Zhou Y, Kong W, et al. 2019. Glutathione activates Type III secretion system through Vfr in Pseudomonas aeruginosa. Front Cell Infect Microbiol. 9: 164.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Beyond pathogenicity: applications of the type III secretion system (T3SS) of Pseudomonas aeruginosa
Tianqi Su, Lin Zhang, Jie Shen, Danyu Qian, Yulei Guo, Zhenpeng Li
Frontiers in Microbiology.2025;[Epub] CrossRef
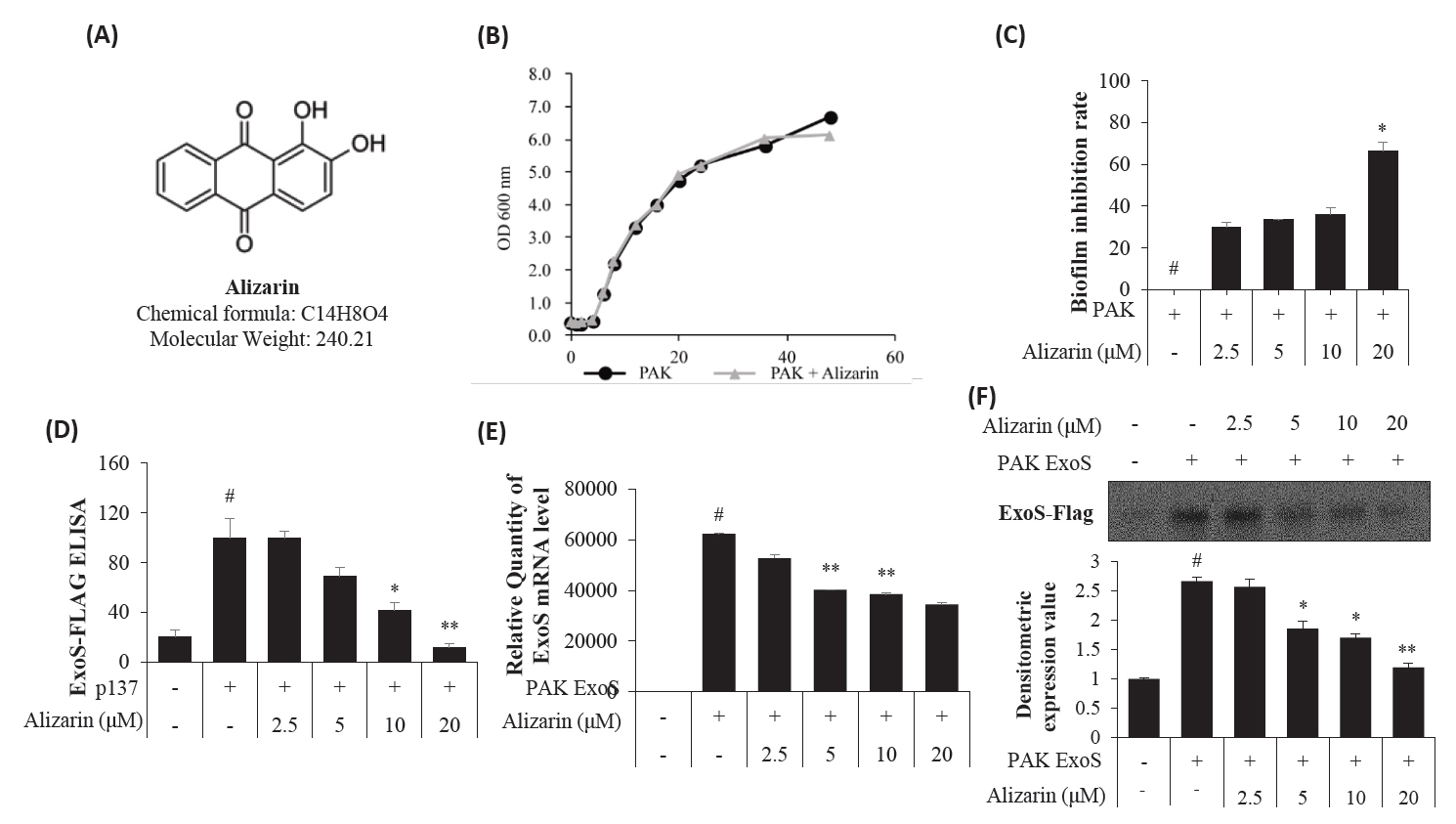
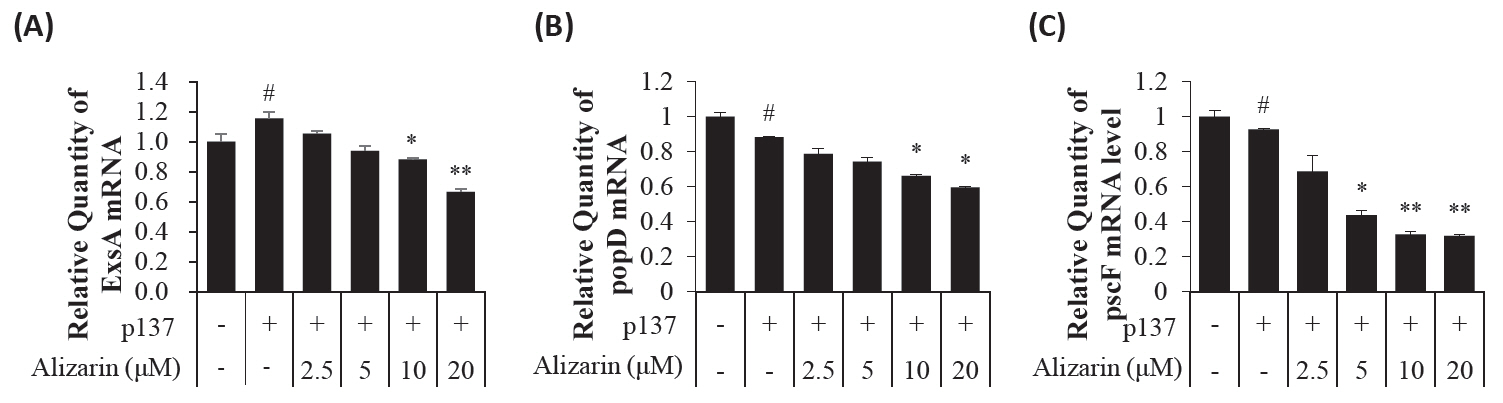
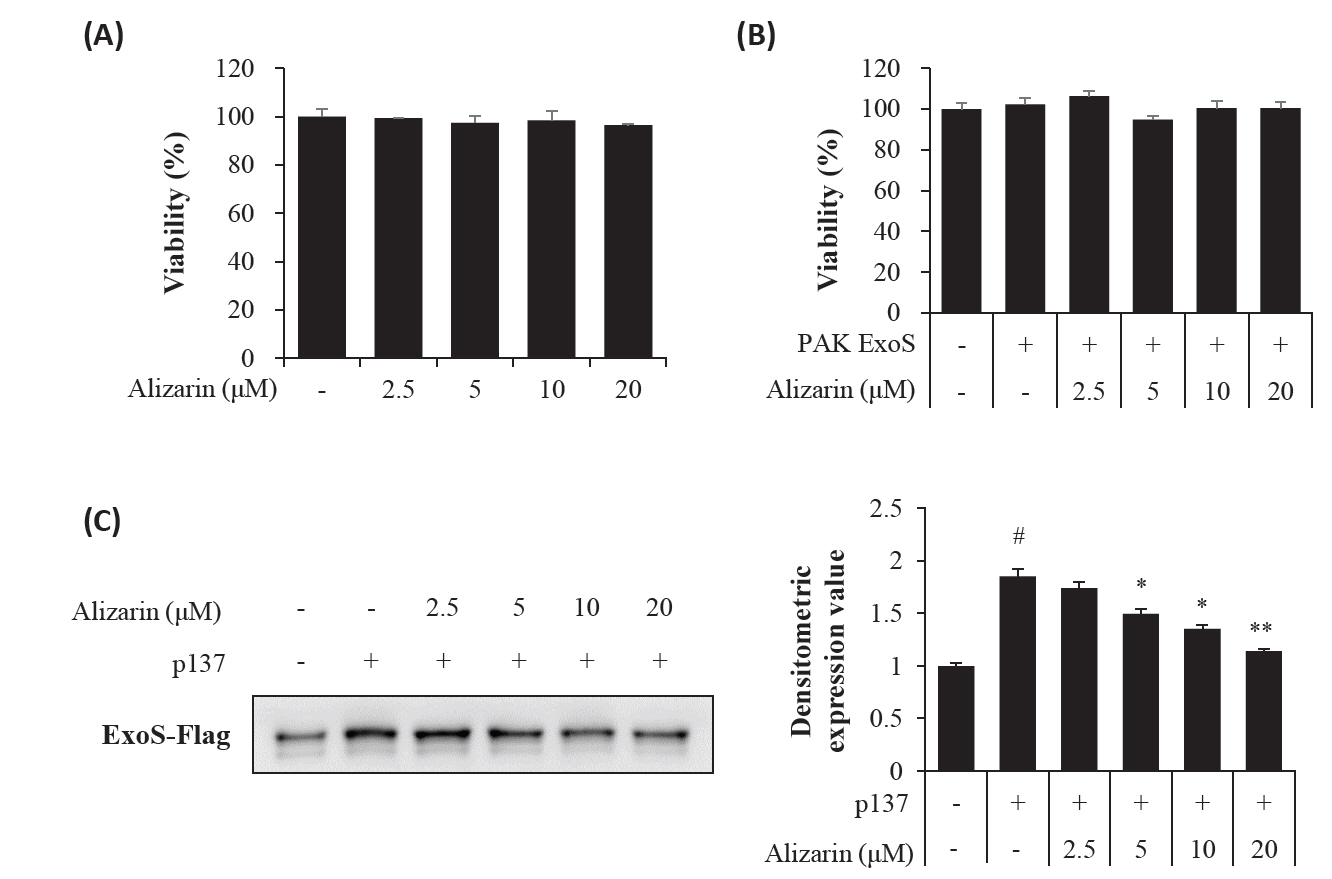
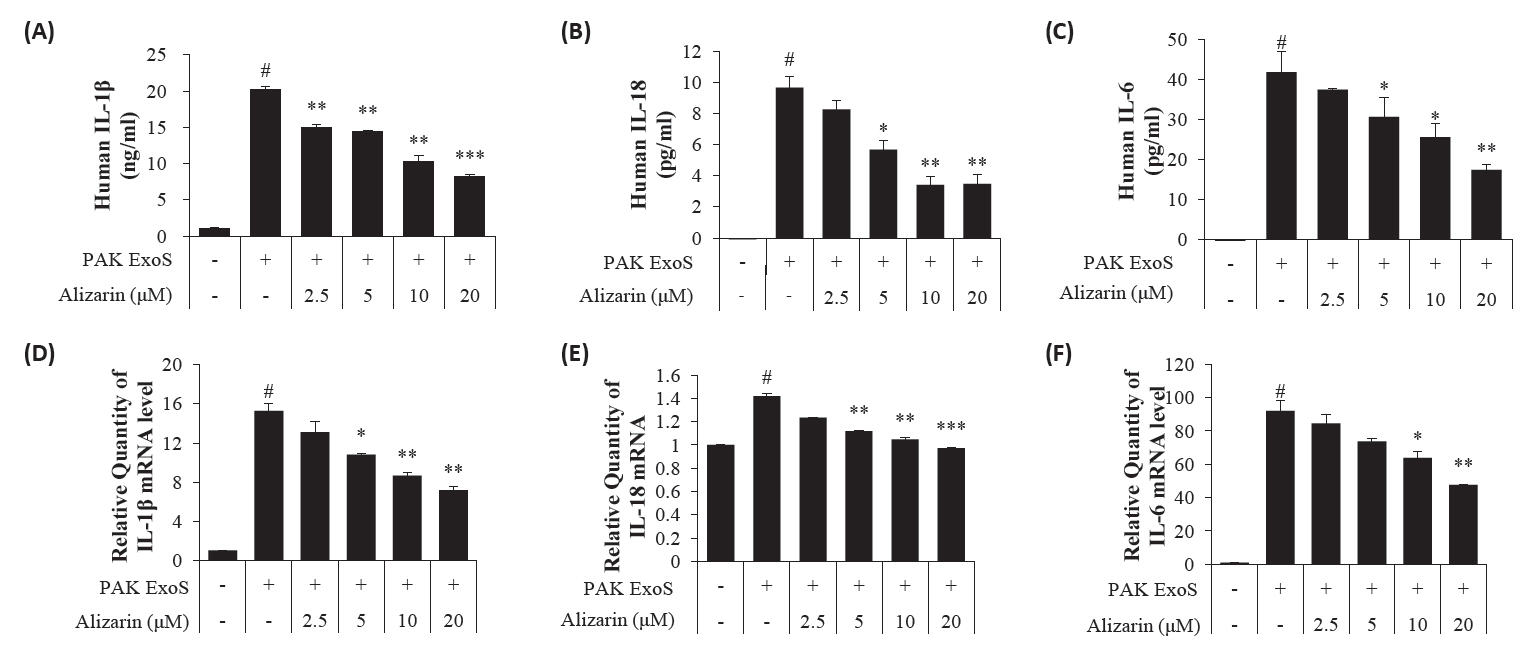
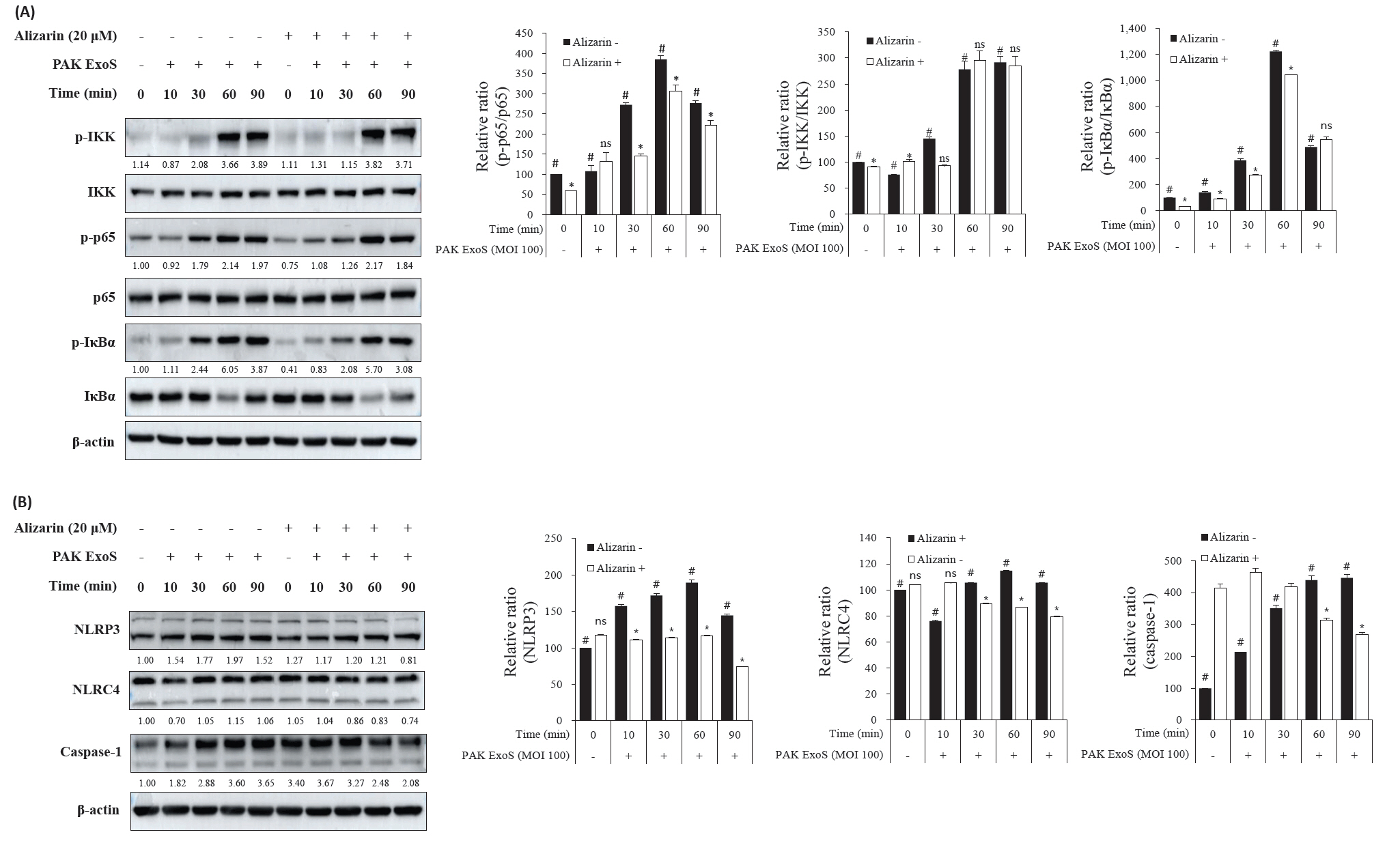
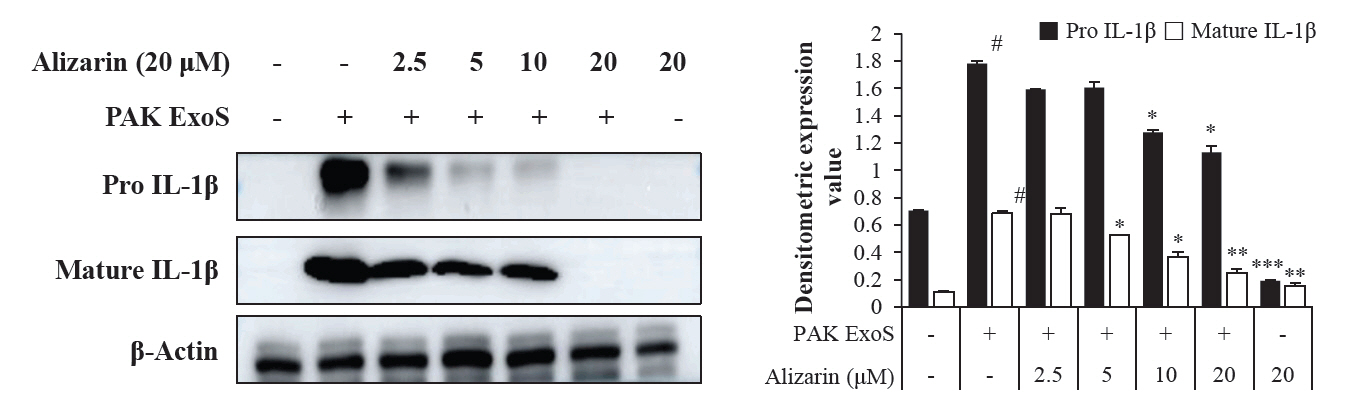
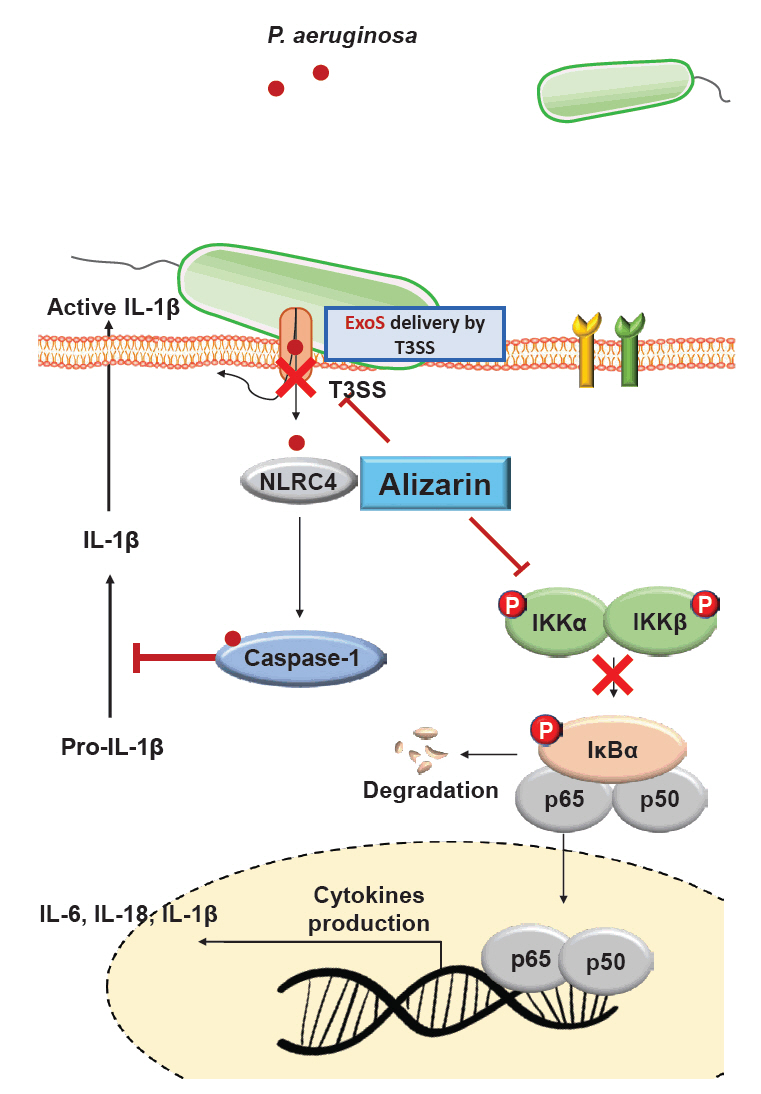
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
| Strain or plasmid | T3SS discription | Antibiotics | Source or reference |
|---|---|---|---|
| Strain | |||
| PAK | Wild type, functional T3SS | University of Florida | |
| PAK exsA::Ω (p34) | PAK with the malfunction of T3SS transcription control gene loci with Ω cassette | Sp200, Sm200, Cb150 | From Dr. Ha (Korea University) |
| PAK-ΔSTmt | Double effector mutant: functional needle and translocon apparatus without known effector | Cb150 | From Dr. Ha (Korea University) |
| PAK-ΔSTmt-pUCP18 (PAK ExoS) | A Functional Effector ExoS In PAK-ΔSTmt | Cb150 | |
| PAK exoST::Ω/pHW0225 (p137) | Double mutated ExoS-FLAG | Sp200, Cb150, Gm150 | From Dr. Ha (Korea University) |
| Plasmid | |||
| PAK-ΔSTmt-pUCP18 | Cloning vector (Vector only 4,557 bp) | Cb150 | From JWP (Natural Medicine Research Center) |
| PAK-ΔSTmt-pUCP18PAKexoS [ExoS] | pUCP18 PAKexoS | From JWP (Natural Medicine Research Center) | |
| PAK-ΔSTmt-pUCP18PAKexoT [ExoT] | pUCP18 PAKexoT | From JWP (Natural Medicine Research Center) |
| Bacteria primer | ||
|---|---|---|
| Forward | Reverse | |
| ExoS | CAG GCT GAA CAG GTA GTG AAG | TTC AGG GAG GTG GAG AGA TAG |
| ExsA | AAG GAG CCA AAT CTC TTG | CTT GTT TAC CCT GTA TTC G |
| popD | ATC CAG TCC TTC GTC CAG AT | TCC TCG ACC TTC TGC TTC T |
| pscF | TCA ACG ACG CGA TCA AGG | CCG TCG AGT TGA TGT TGT AGA T |
| 16S | CAA AAC TAC TGA GCT AGA GTA CG | GCC ACT GGT GTT CCT TCC TA |
| Human primer | ||
| IL-1β | GGG CCT CAA GGA AAA GAA TC | TTC TGC TTG AGA GGT GCT GA |
| IL-18 | TGC ATC AAC TTT GTG GCA AT | ATA GAG GCC GAT TTC CTT GG |
| IL-6 | GAC AGC CAC TCA CCT CTT CA | AGT GCC TCT TTG CTG CTT TC |
| gapDH | CCC TCC AAA ATC AAG TGG | CCA TCC ACA GTC TTC TGG |
| IL-8 | ACT GAG AGT GAT TGA GAG TGG AC | AAC CCT CTG CAC CCA GTT TTC |
Table 1.
Table 2.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article








