ABSTRACT
-
Streptococcus pneumoniae is a conditionally pathogenic bacteria that colonizes the nasopharynx of 27% to 65% of children and 10% of adults. Capsular polysaccharides are the most critical virulence factor of S. pneumoniae, and nonencapsulated strains are usually non-pathogenic. Previous studies have shown that glucose regulates capsule synthesis. To investigate the mechanism of carbon metabolism regulatory factors CcpA and HPr regulating capsule synthesis in the presence of glucose as the sole carbon source, we constructed deletion mutants (D39ΔccpA and ΔptsH) and complemented strains (D39ΔccpA::ccpA and ΔptsH::ptsH). In this study, we found that the promoting effect of capsule synthesis by glucose disappeared after the deletion of ccpA and ptsH, and demonstrated that the protein CcpA regulates capsule synthesis by binding to the cps promoter and altering the transcription level of the cps gene cluster. Increased glucose concentration up-regulated the level of HPr-Ser46~P, which enhanced the binding ability of CcpA to the DNA sequence of the cps promoter, thus promoting capsule synthesis. HPr also has a regulatory effect on capsule synthesis. These insights reveal a new synthesis mechanism of capsular polysaccharide and provide a new strategy of antibacterial drugs for S. pneumoniae.
-
Keywords: Streptococcus pneumoniae, capsular polysaccharide, CcpA, glucose, HPr
Introduction
Streptococcus pneumoniae is a Gram-positive bacteria that colonizes the nasopharynx extensively and is transmitted primarily by inhalation of infectious aerosols or direct contact with infected mucous membranes or secretions. It can invade and infect multiple organs, including the middle ear, lungs, bloodstream, heart, and brain, often resulting in mucosal infections and invasive infections in both children and adults (Dao and Rosch, 2021). S. pneumoniae colonization and infection are endemic to all regions and are the leading cause of pneumonia deaths worldwide (Troeger et al., 2018), with more than 1.5 million fatalities each year attributed to invasive and fatal pneumococcal disease (Butters et al., 2019; Wahl et al., 2018). Traditional treatments for S. pneumoniae infection include antibiotic therapy and vaccines. However, current studies have shown that S. pneumoniae has developed resistance to a variety of antibiotics, including β-lactams, macrolides, and tetracyclines (Zhou et al., 2022). To address the diversity of capsular polysaccharides and prevent infections caused by multiple serotypes, the development of vaccines is crucial. Unfortunately, commercially available vaccines still have limitations, such as restricted serotype coverage and serotype substitution (Weinberger et al., 2011), so there is an urgent need to find new targets for the development of antimicrobial drugs.
Capsular polysaccharides (CPS) is the major virulence factor required for effective colonization and induction of invasive disease (de Vos et al., 2015; Hyams et al., 2010; Wartha et al., 2007). Nonencapsulated strains are usually non-pathogenic. Therefore, to explore the synthetic regulatory mechanism helps to find new antibacterial targets.
Current studies have identified 109 serotypes in S. pneumoniae (Su et al., 2021). Most CPS is synthesized via the Wzx/Wzy-dependent pathway, and the genes involved in CPS synthesis are located between dexB and aliA, except for serotype 37 (Geno et al., 2015; Yother, 2011). cps gene cluster is involved in the formation of CPS and its expression is driven by a single cps promoter (Shainheit et al., 2014). It has been reported that the transcript levels of cps genes can be affected by deletion of individual elements between the coding sequences of dexB and cps2A (Wen et al., 2015). Bioinformatics analysis showed that there were motifs similar to transcription regulatory binding sites in the promoter region of cps gene cluster, such as RitR, MalR, CopY, and AdcR, which need to be confirmed by relevant experimental evidence (Moscoso and Garcia, 2009). CpsR and phosphorylated ComE have been confirmed to bind specifically to the cps promoter sequence and negatively regulate CPS synthesis (Wu et al., 2016; Zheng et al., 2017).
Several environmental factors, such as oxygen availability (Geno et al., 2014) and carbon sources (Troxler et al., 2019), have been associated to regulate cps gene transcription. CcpA (Catabolite Control Protein A) is a key regulator of carbon metabolite repression. With the help of the phosphoenolpyruvate-dependent phosphotransferase system (PTS), it binds to the specific DNA sequence to promote or repress the expression of the target gene, thereby regulating the physiological process and virulence of bacteria (Warner and Lolkema, 2003; Yang et al., 2017). Previous studies have confirmed that CcpA has a significant impact on the capsule synthesis and virulence in Streptococcus suis, with the absence of its activity leading to reduced capsule expression and decreased resistance to phagocytosis and killing (Willenborg et al., 2011). In S. pneumoniae, the RegM mutation, an analog of CcpA, resulted in a significant reduction in the transcription of cps gene cluster and a decrease in virulence (Giammarinaro and Paton, 2002). Our previous studies have demonstrated that CcpA not only affects the natural transformation by regulating the transcription of comCDE, but also promotes CPS synthesis in S. pneumoniae (Zhang et al., 2023). Therefore, we proposed to explore whether CcpA can sense the sugar signal in S. pneumoniae and regulate CPS synthesis accordingly.
HPr is a component of PTS encoded by the ptsH gene. During sugar uptake, serine residues of HPr protein are phosphorylated by HPr kinase/phosphatase (HprK/P) to form HPr-Ser46~P and produce a complex with CcpA, which is involved in CCR effects (Chauvaux, 1996). Research has shown that HPr-Ser46~P is not only a central regulator of carbon metabolism, but also plays an significant role in the virulence development of Gram-positive bacteria such as Streptococcus pyogenes, Bacillus subtilis, and Listeria monocytogenes (Deutscher et al., 2005). However, to data, no study has reported whether HPr-Ser46~P affects the regulatory effects of CcpA on CPS synthesis in S. pneumoniae.
In this study, we describe the specific ways in which CcpA regulates CPS synthesis in an environment where glucose is the sole carbon source, and explore how S. pneumoniae modulates CPS synthesis through CcpA in response to different concentrations of glucose. This regulatory mechanism that requires the presence of the auxiliary protein HPr, which is altered by activation or inhibition of environmental signals, resulting in changes to its phosphorylation levels. These changes subsequently affect the activity of CcpA binding to the cps promoter, ultimately influencing CPS synthesis. This study elucidates the molecular mechanism of glucose-regulated CPS synthesis through CcpA and HPr, and provides a new target and rationale for the development of new therapeutic approaches for S. pneumoniae disease.
Materials and Methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table S1. All S. pneumoniae strains were grown at 37°C under 5% CO2 in semi-synthetic casein hydrolyzate medium supplemented with 5% yeast extract (C + Y, pH 7.0) medium or plated on blood agar plates. Escherichia coli strains were grown in lysogeny broth (LB) with shaking or LB agar plates at 37°C. Selective antibiotics were added when necessary.
Bacterial strains constructions
The primers used in this study are listed in Table S2. We constructed mutants by generating unlabeled mutations in the pneumococcal genome using the Janus technique. All mutants are derived from the D39s strain, a streptomycin-resistant derivative of D39ΔptsH was produced in a two-step transformation process. First the upstream and downstream sequences of the ptsH locus were amplified with primer pairs Pr1440/1441 and Pr1442/1443 from D39s. The Janus cassette was amplified with primers Pr1332 and Pr1333 from genomic DNA of strain ST588. These amplicons were ligated to obtain a recombinant fragment that was transformed into D39s to construct ΔptsH::kana-rpsL (ΔptsH::JC). Unmarked deletions in the ptsH locus were then constructed by overlapping upstream (primers Pr1440/YR0013) and downstream (primers Pr1443/YR0014) sequences with primers Pr1440/1443 and transformed into strain ΔptsH::JC to construct unmarked deletion strain (ΔptsH).
The ptsH complemented strain was constructed by using shuttle plasmid pIB166. DNA fragments containing the ptsH gene and its predicted upstream promoter are amplified by PCR with primer YR0019/YR0020. the obtained DNA fragments were digested with suitable restriction enzymes (BamHI and XhoI) and cloned into the shuttle plasmid pIB166 to generate the recombinant. The recombinant plasmids pIB166-ptsH was transformed into the ΔptsH mutant strain to construct the ptsH complemented strain (ΔptsH::ptsH).
Recombinant protein production
The pneumococcal ptsH gene from S. pneumoniae D39 was amplified by PCR and cloned into the pET-28a expression vector and into E. coli BL21 (DE3). Expression was induced at an OD600 of 0.8 by addition of 1 mM isopropyl-D-thiogalactoside (IPTG) at 20°C by shaking at 120 rpm for 8–10 h. The HPr protein was collected and purified by affinity chromatography with an Ni21/nitrilotriacetic acid (NTA) column. To obtain HPr-Ser46~P protein, we mutated the 136th serine residue (TCA) to aspartic acid residue (GAC) in the ptsH gene. The point mutation fragment was confirmed by sequencing and cloned into the pET-28a expression vector and into E. coli BL21 (DE3). Subsequent steps were produced in the same way.
Uronic acid assay
Bacterial strains were cultured to OD600 = 0.5 at 37°C and 5% carbon dioxide in C + Y medium to collect 3ml and centrifuged at 4°C and 12,000 rpm for 5 min, removing the supernatant and leaving the pellet. Bacterial precipitations were resuspended in 500 μl of Tris-HCl/MgSO4 buffer (containing 150 mM Tris·HCl and 1 mM MgSO4, pH 7.0). The samples were added 2 μl sulfamate and 1 ml of borate solution, heated at 100℃ for 15 min, cooled to room temperature, and then added 16 μl m-hydroxybiphenyl solution (0.15%), placed for 30 min, the amount of capsules was detected by measuring absorbance at 525 nm.
Western blot
Bacterial strains were grown in C + Y medium at 37°C to an OD600 of 0.5. The samples were centrifuged, and the cell pellets were lysed with lysis buffer (0.5% deoxycholate). The protein concentration was measured using the NanoDrop spectrophotometer, and the samples were subjected to SDS-PAGE and electrotransferred onto 0.2 mm polyvinylidene difluoride (PVDF) membranes (Merck Millipore, USA). To detect HPr, purified HPr was used to immunize kunming mice to generate polyclonal antibodies that was used diluted at 1:5000. To detect capsule, Pneumococcus Type 2 serum was used diluted at 1:5000. The proteins were visualized by adding Immobilon Western horseradish peroxidase (HRP) substrate peroxide solution (Millipore) captured using Image Lab software (Bio-Rad).
Quantitative real-time PCR
According to the operating manual, RNA was extracted using the RNAprep Pure Cell/Bacteria Kit (Tiangen). Reverse transcription reactions were performed using the prime script RT Master Mix kit (TaKaRa-Bio). Quantitative PCR reactions were performed using the BioRad CFX Connect PCR instrument, and the primers were quantified as shown in Table S2. Relative changes in gene expression were calculated by the 2-ΔΔCt method using gyrB as an internal reference gene. The results of representative experiments are presented as the means of three replicates ± Standard Error of the Mean.
Electrophoretic mobility shift assay
The 5′-biotin-labeled and unlabeled DNA fragments containing the promoter region of the Pcps probes was amplified from strain D39. Chemiluminescence EMSA kits were purchased from Beyotime. EMSAs are carried out according to the manufacturer’s instructions. The EMSA reaction was performed in a total volume of 10 μl, followed by the addition of ddH2O, 5X binding buffer, different concentrations of protein and unlabeled probes. The reaction mixture was incubated for 10 min at room temperature. After incubation, labeled probe was added and the reaction mixture was incubated for 20 min at room temperature. Subsequently, 10 μl of the solution was loaded onto a precast 6% acrylamide gel (Invitrogen) and electrophoresis at 100 V for 60 min. DNA was transferred to a nylon membrane and crosslinked by exposure to ultraviolet (UV) light, and the bands were visualized with a chemiluminescence substrate and captured using the Chemiluminescence imaging system (Bio-Rad).
Luciferase activity assay
Luciferase reporter construct of the cps promoter region was from Tsinghua University. The plasmid pTH3937 was transferred to D39s, ΔccpA and ΔptsH to generate D39s-Pcps-luc, D39sΔccpA-Pcps-luc and D39sΔptsH-Pcps-luc. The relative luminescence unit (RLU) values were determined in a GloMax luminescence detector (Promega) by adding 1 μl D-fluorescein (0.66 mM) (Beyotime) to a 100 μl bacterial medium. The optical density (OD600) of the samples was measured and used to normalize the luciferase activity.
Animal experiments
Female C57BL/6 mice (6–8 weeks old, weighing 20–22 g) were purchased from the Laboratory Animal Center of Chongqing Medical University. The Ethics Committee of Chongqing Medical University approved this study.
D39s, ΔptsH and ΔptsH::ptsH strains were grown to 0.5 in C + Y, bacteria were collected by centrifugation, washed and suspended in sterile PBS. For the lung infection model, C57BL/6 mice (6 to 8 weeks old, female) were infected intratracheally with 2 × 107 CFU. For pneumonia model and survival assay, C57BL/6 mice (6 to 8 weeks old, female) were infected intratracheally with 1 × 108 CFU, mouse survival was monitored daily for 14 days. To determine the organ involvement, blood aliquots and nasal lavage samples were collected from mice following induction of general euthanasia at 48 h after infection. Lung and spleen whole tissues were homogenized. Bacterial counts in the blood as well as organ homogenates were determined by separately plating serial dilutions. The results were presented as the means of three replicates ± the standard deviation (SD).
Statistical analysis
The survival was analyzed using the log-rank test. All others were analyzed using two-tailed unpaired Student’s test, with statistical significance defined as P < 0.05 (*), < 0.01 (**) and < 0.001 (***).
Results
CcpA promotes CPS synthesis in an environment with glucose as the sole carbon source
CcpA is a central regulator of the carbon catabolite repression (CCR) and previous studies have shown that CcpA promotes CPS synthesis (Zhang et al., 2023), so we proposed to investigate whether CcpA is able to sense sugar signals to regulate CPS synthesis. Since glucose is the preferred carbon source for most microorganisms, we mainly explored the molecular mechanism by which single glucose regulates CPS via CcpA.
To clarify the effect of CcpA on CPS synthesis in an environment with glucose as the sole carbon source, Western blot was performed to determine the production of CPS in D39s, ΔccpA, and ΔccpA::ccpA in C + Y medium with 10mM glucose as the sole carbon source. The result revealed that the production of CPS in ΔccpA was significantly reduced compared to the wild-type (Fig. 1A). Uronic acid assay also showed a consistent trend (Fig. 1B). These results indicated that CcpA has a promoting effect on CPS synthesis in an environment with glucose as the sole carbon source.
Since CPS is the most important virulence factor of S. pneumoniae (Su et al., 2021), inhibition or interference with CPS synthesis significantly attenuates the virulence of S. pneumoniae, we assessed the adhesion and antiphagocytic ability of D39s, ΔccpA, and ΔccpA::ccpA. There was a significant increase in the amount of ccpA-deficient strain adhering to the macrophage surface or being engulfed into the macrophage. The result coincided with the changes in CPS content, corroborating the positive regulation of CPS synthesis by CcpA (Fig. 1C and 1D).
CcpA binds to the cps promoter to promote transcription of the cps operon
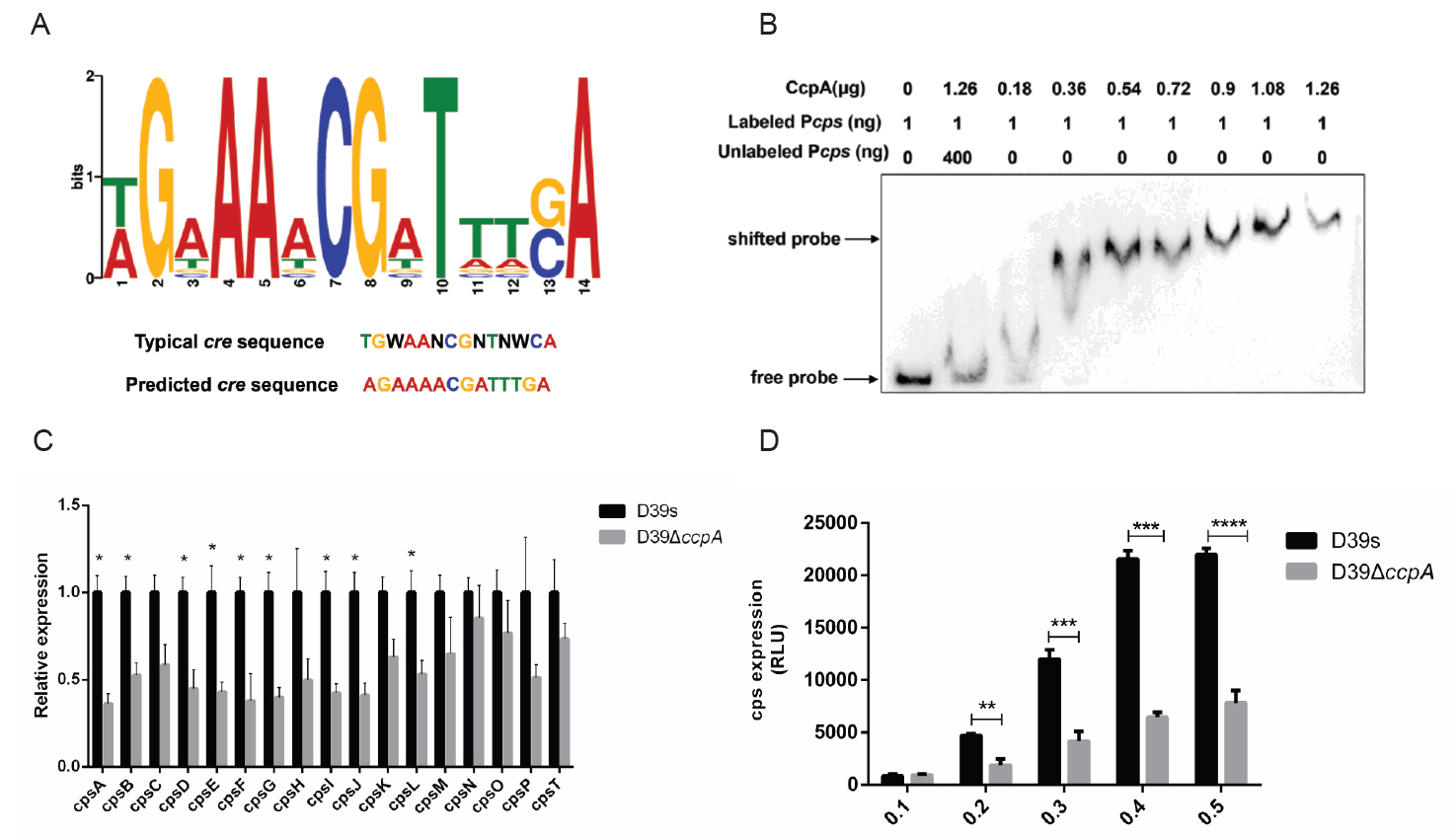
CcpA is a transcriptional regulator that belongs to the LacI/GalR family (Henkin, 1996). Studies have shown that CcpA affects gene expression by binding to cis-regulated DNA known as catabolite responsive elements (cre), which consist of a typical consensus site, TGWAANCGNTNWCA (Weickert and Chambliss, 1990). We identified a conserved CcpA-binding sequence (Fig. 2A) in the cps promoter region. To further determine whether CcpA specifically binds to the cps promoter, we expressed and purified CcpA protein (Fig. S1A), and detected binding ability by gel mobility shift assay (EMSA). As CcpA concentration increased, a significant increase in the DNA-protein complex and the binding of CcpA to the labeled probe was inhibited by adding a non-biotin-labeled competing probe (Fig. 2B). The result indicated the specific binding of CcpA to the cps promoter.
Q-PCR was performed to test the expression of several genes in the cps locus. The results showed that the expression of genes in ccpA-deficient bacteria were all down-regulated compared to the wild-type (Fig. 2C). We further assessed the effect of CcpA on cps operon transcription by investigating the activity of cps promoter in luciferase reporter strains. Consistent with the Q-PCR results, the luciferase activity of D39ΔccpA-Pcps-luc at different growth stages was lower than that of D39s-Pcps-luc significantly (Fig. 2D). These results suggested that CcpA may regulate CPS synthesis by specifically binding to the cps promoter and promoting the expression of cps operons.
CcpA is involved in the regulation of glucose on capsule synthesis
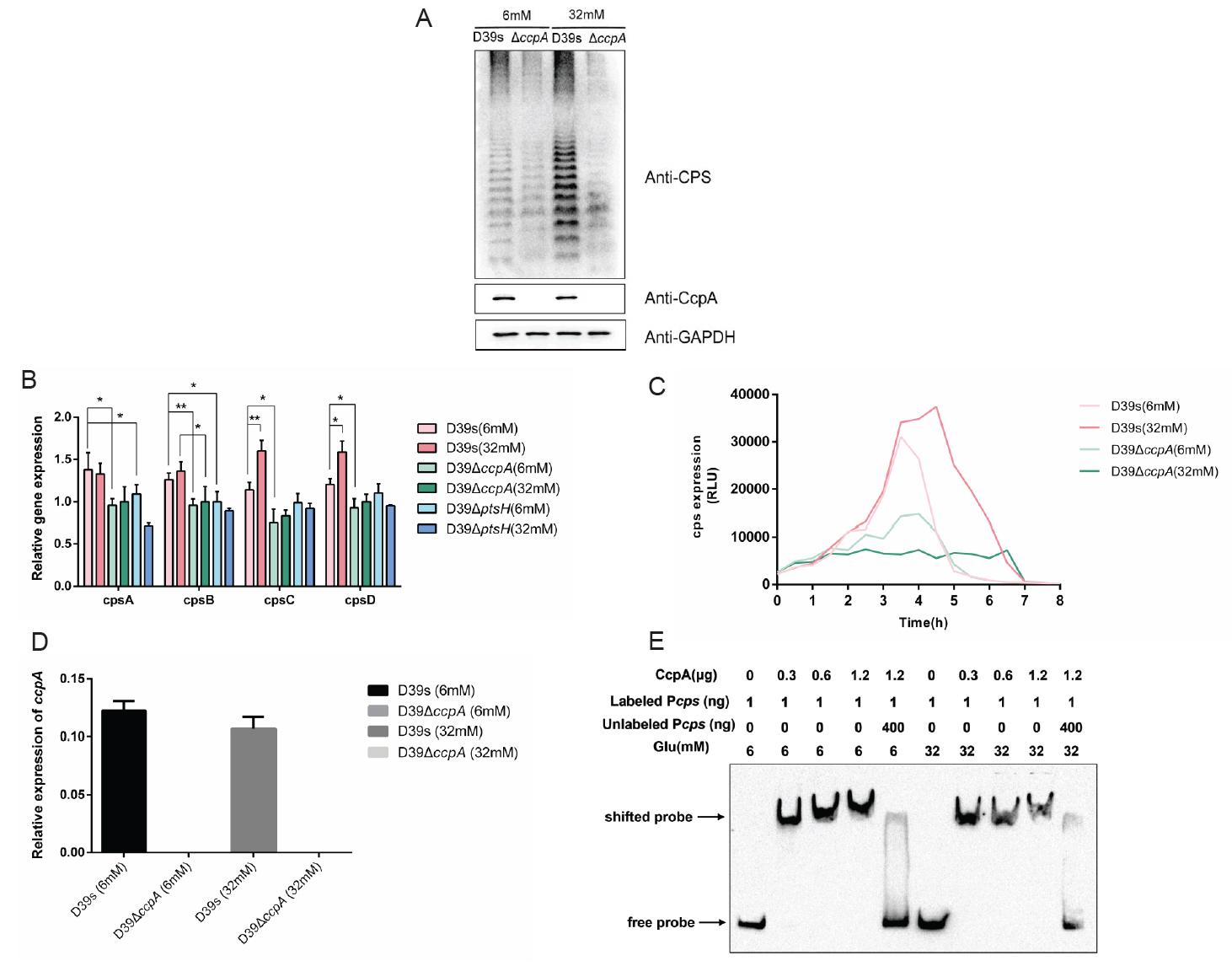
To investigate whether glucose regulates the transcriptional levels of genes in the cps locus by CcpA, we examined the CPS contents and the mRNA expression of the first four genes of the cps operon (cps2A–2D) in wild-type and ccpA-deficient strains at 6 mM (low concentration) and 32 mM (high concentration) glucose concentrations, respectively. The result showed that differences in CPS content at different glucose concentrations diminished upon CcpA deficiency (Fig. 3A), and steady-state mRNA levels in wild-type strains for cps2C/D was increased in high glucose environment compared to low glucose environment. In the ccpA-deficient strains, there was no significant difference at different glucose concentrations, and mRNA levels were down-regulated in ccpA-deficient strains compared with wild-type at a low glucose concentration (Fig. 3B). We further assessed the activity of cps promoter. The result indicated that the cps promoter activity of the wild-type increased with increasing glucose concentration, whereas was unchanged in ΔccpA mutant. Furthermore, cps promoter activities were significantly lower in ccpA-deficient strains compared to wild-type (Fig. 3C). In conclusion, increased glucose concentration promoted cps operons transcription, and this regulation required the involvement of CcpA.
Therefore, we further explored how glucose affect CcpA-mediated regulation. However, the mRNA expression of ccpA remained unchanged (Fig. 3D) and its protein levels had no difference at different glucose concentrations (Fig. 3A). In addition, we found that variations in glucose concentration did not affect the binding of CcpA to the cps promoter (Fig. 3E). These results suggested that glucose regulated the effect of CcpA on CPS synthesis in other ways.
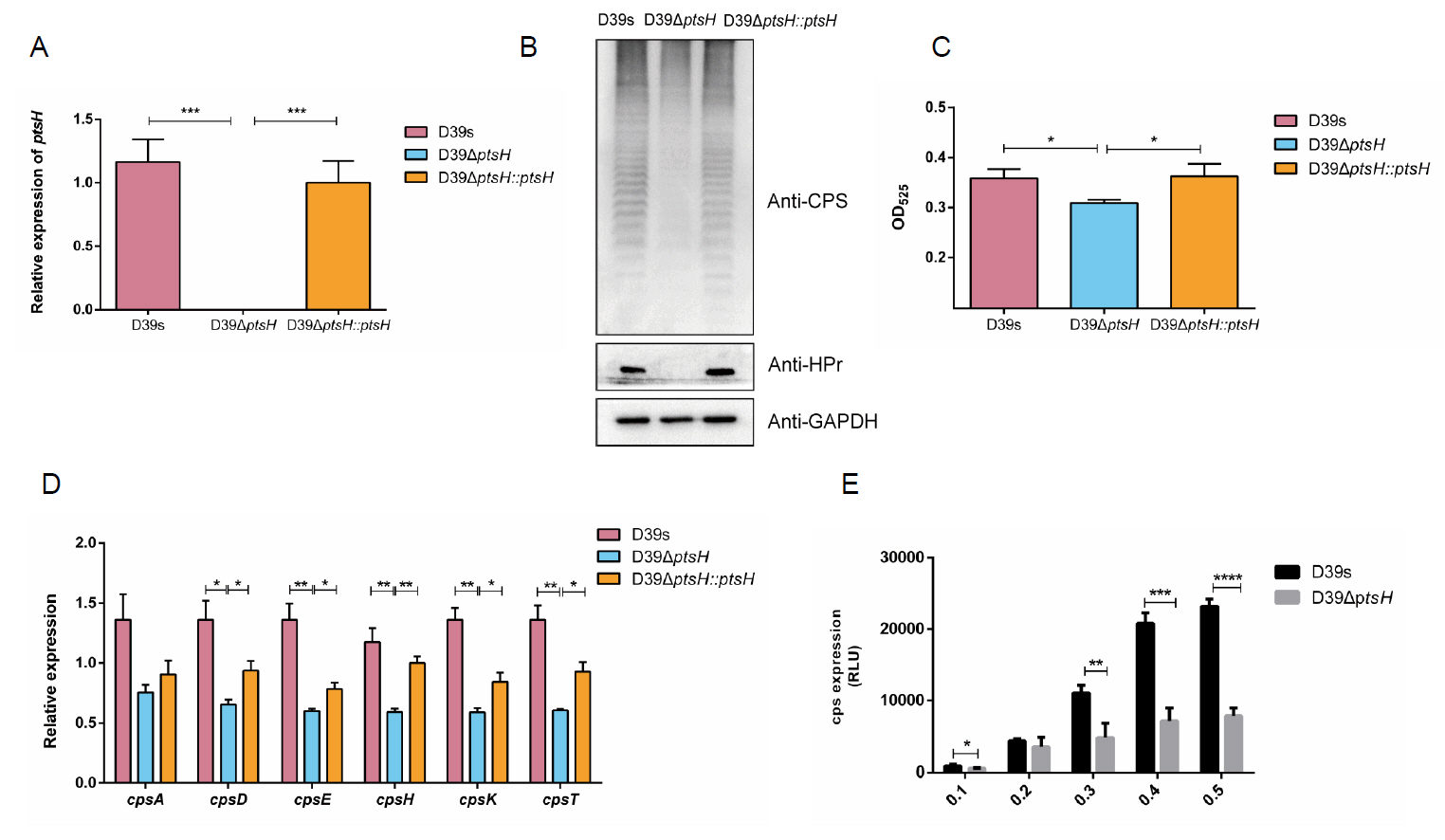
HPr promotes capsule synthesis
HPr is a synergistic effector of CcpA that affect CcpA activity by binding to the cre site (Fujita, 2009; Görke and Stülke, 2008). To investigate whether HPr has a regulatory effect on CPS synthesis, we constructed ptsH silent mutation (ΔptsH) in the streptomycin-resistant D39-derived strain (D39s) by using the Janus cassette (JC) (Sung et al., 2001), the complemented mutant ΔptsH::ptsH was generated by transforming the shuttle plasmid pIB166-ptsH into ΔptsH mutant. Q-PCR and Western bolt analysis showed that the mRNA and protein were absent in ΔptsH mutant and restored in ΔptsH::ptsH (Fig. 4A and 4B). These results indicated that ptsH -deficient and ptsH -complementary strains were successfully constructed. Next, Western blot was performed to determine the production of CPS in D39s, ΔptsH, and ΔptsH::ptsH in normal C + Y medium. The result revealed that the content of CPS in ΔptsH mutant was significantly reduced compared to the wild-type (Fig. 4B). To further confirm the western blot result, we quantified the amount of CPS of the strains by uronic acid assay. The uronic acid level of ΔptsH was significantly lower than that of D39s, while the content was completely restored in the complemented strain ΔptsH::ptsH (Fig. 4C), suggested that HPr promoted CPS synthesis.
CPS is a key virulence factor of S. pneumoniae. Mouse infection experiments were performed. We intranasally infected mice (pneumonia model) using 108 CFU bacteria, and two days after infection, mice infected with D39s and ΔptsH::ptsH began to die, with a nearly 50% reduction in survival, whereas almost all mice infected with ΔptsH survived (Fig. S2A). In a low-dose pneumonia infection model (2 × 107 CFU), we collected nasal lavage, blood, lung and spleen homogenates from mice in different infection groups 48 h after infection for plate counting, and the bacterial load in the ΔptsH group was significantly lower than that in both the D39s and ΔptsH::ptsH groups (Fig. S2B, S2C, S2D, and S2E). These results suggested that the deletion of ptsH resulted in a significant reduction in virulence.
Next, we examined the mRNA levels of cps2A, cps2D, cps2E, cps2H, cps2K, and cps2T in D39s, ΔptsH, and ΔptsH::ptsH strains. The mRNA levels of ΔptsH were down-regulated relative to D39s, whereas were partially restored in the complemented strain ΔptsH::ptsH (Fig. 4D). Additionally, we constructed the luciferase reporter strain D39ΔptsH-Pcps-luc to observe the effect on cps promoter activity. The result showed that deletion of ptsH resulted in a significant reduction in promoter activity (Fig. 4E). These results suggested that HPr positively regulated CPS biosynthesis by influencing the transcription of the cps operon.
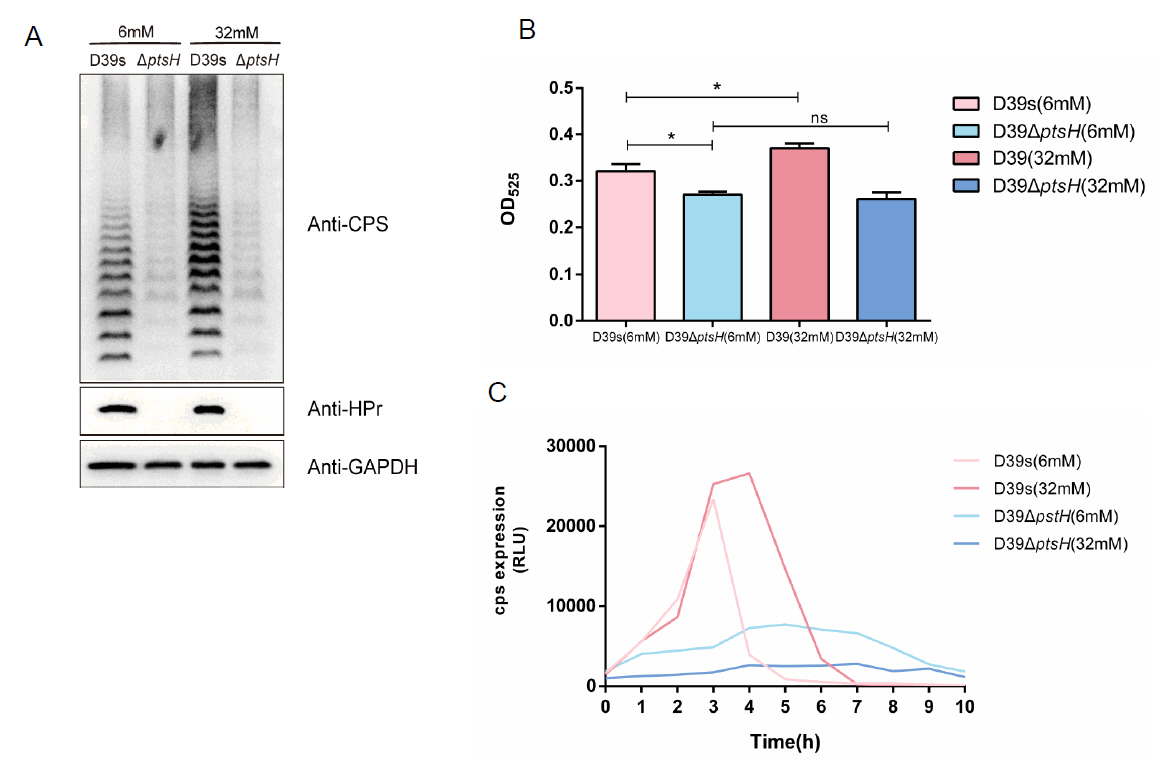
HPr is involved in the regulation of glucose on capsule synthesis
To investigate whether HPr is also involved in the regulation of CPS synthesis by glucose, we performed Western blot (Fig. 5A) and uronic acid assay (Fig. 5B) on D39s and ΔptsH grown in C + Y medium supplemented with 6 mM and 32 mM glucose concentrations, which showed that, similar to ΔccpA, there was almost no difference in the CPS content of ΔptsH in different glucose concentrations. we also assessed the transcript levels of the cps2A, cps2B, cps2C, and cps2D genes (Fig. 3B), the expression of the genes was down-regulated and did not increase with higher glucose concentration in ΔptsH compared to wild-type. the same trend was also shown in the activity assay of the cps promoter (Fig. 5C). These results suggested that HPr was involved in the glucose regulation of CPS.
Glucose promotes the formation of HPr-Ser46~P to promoting the regulation of CcpA on the capsule
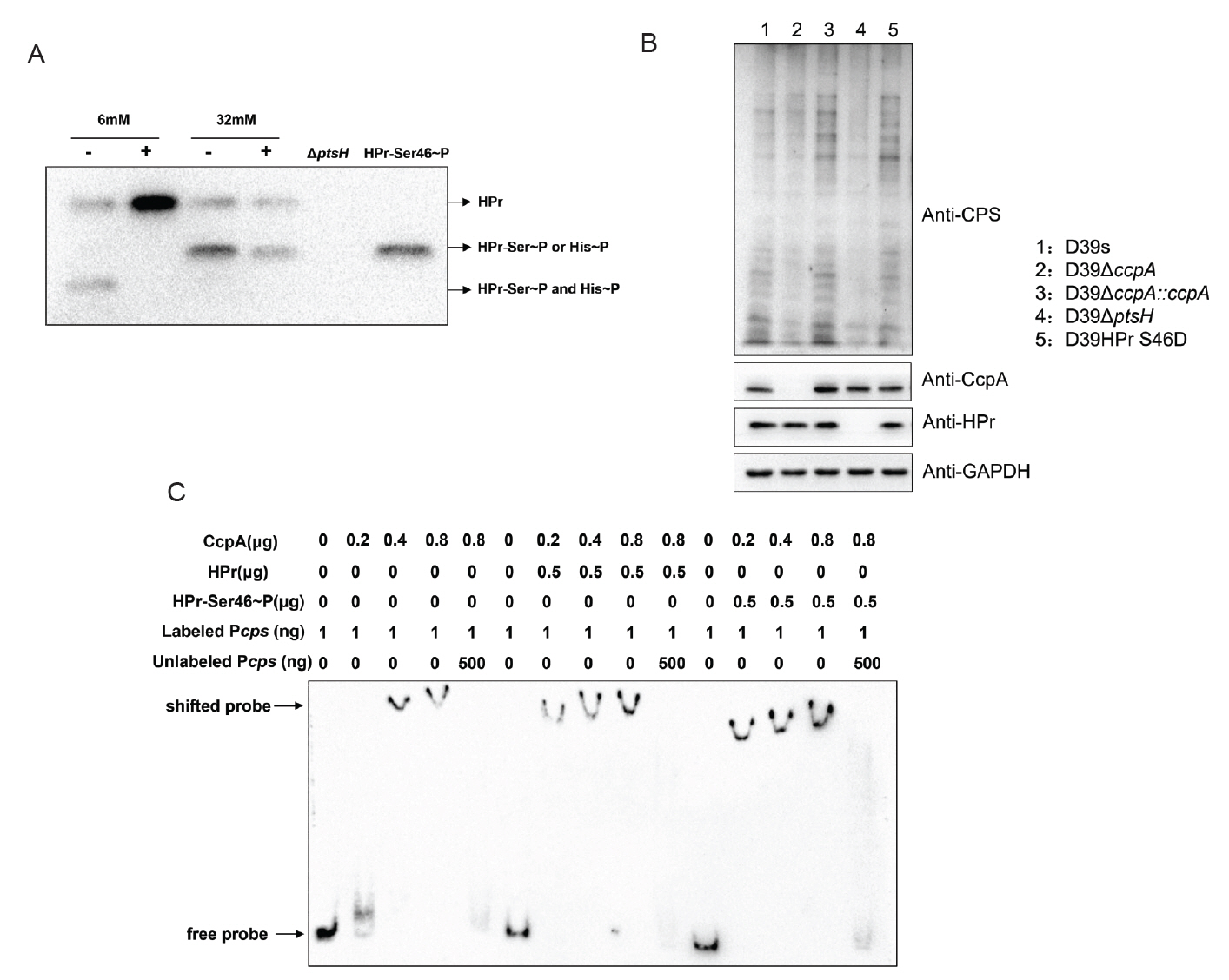
It has been proposed that in group A streptococci (GAS), HPr is able to sense extracellular glucose concentration to phosphorylate or dephosphorylate itself, then to bind or dissociate from CcpA, which leads to changes in the binding of CcpA to cre (Shelburne et al., 2008). Studies reported that in B. subtilis, CcpA specifically interacted with HPr-Ser46~P to play a regulatory role, whereas dephosphorylated HPr, HPr-His15~P, and bisphosphorylated [HPr(Ser46~P)(His15∼P)] did not bind to CcpA (Deutscher et al., 1995). Therefore, we hypothesized that in S. pneumoniae, glucose may also affect CcpA-mediated regulation by influencing HPr-Ser46~P.
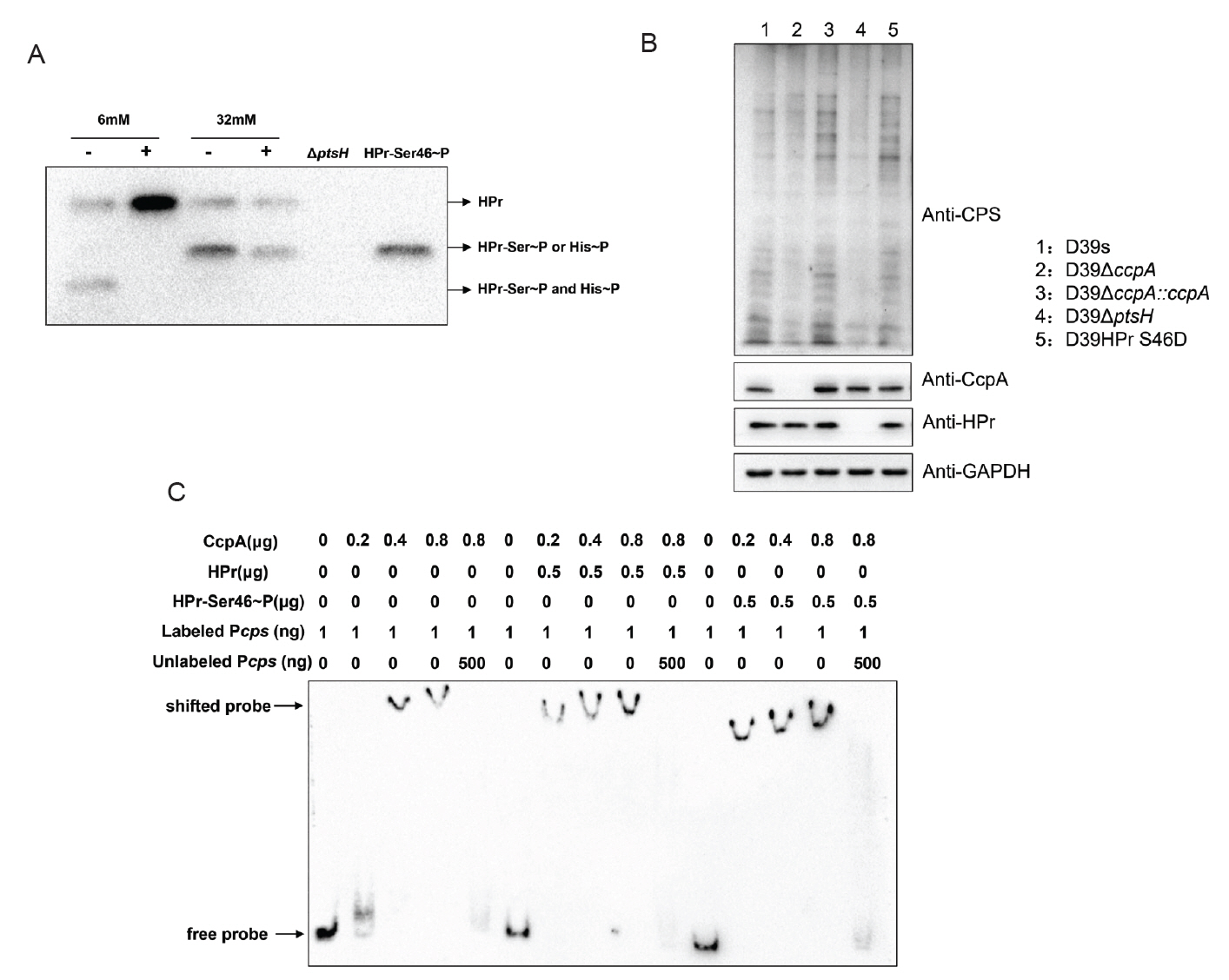
To verify the above conjecture, we performed immunoblotting with anti-S. pneumoniae HPr antibody to determine the phosphorylation status of HPr in wild-type strains grown at different glucose concentrations. Since the amino phosphate bond of HPr-His15~P is thermally unstable, whereas the phosphate bond of HPr-Ser46~P is stable (Roy et al., 2008), we distinguished HPr-His15~P and HPr-Ser46~P that migrated to the same location by boiling the samples. The result showed that wild-type strains growing at a low glucose concentration (6 mM) had distinct bands corresponding to the double phosphorylated form of HPr [HPr (Ser46~P)(His15~P)] and dephosphorylated HPr, whereas strains grown at high glucose concentration (32 mM) were predominantly monophosphorylated forms of HPr-His15~P and HPr-Ser46~P (Fig. 6A).
We further attempted to construct serine residue mutations of HPr, which are commonly used to study HPr-mediated CCR in Gram-positive bacteria: HPr S46A and HPr S46D, but HPr S46A, the HPr mimic dephosphorylation mutation, is lethal in S. pneumoniae (Fleming et al., 2015). we only successfully constructed HPr S46D simulated phosphorylation mutant and investigated its effect on CPS synthesis. Western blot result showed that the CPS content of the HPr S46D strain was significantly enhanced compared with D39s, indicating that increased phosphorylation of HPr-Ser46~P indeed promoted CPS synthesis (Fig. 6B).
Since the literature suggested that HPr-Ser46~P may affect the binding of CcpA to the promoters of target genes, we examined the ability of CcpA protein to bind to the cps promoter in the absence or presence of HPr and HPr-Ser46~P proteins (Fig. 6C). The result showed that both HPr and HPr-Ser46~P significantly enhanced the binding of CcpA to the cps promoter, with the latter having a higher enhancement, suggesting that HPr-Ser46~P can promote the binding of cps promoter to CcpA more strongly than the prototype.
Overall, the increase in glucose concentration promoted the formation of HPr-Ser46~P, which facilitated the binding of CcpA to the cps promoter. This coordinated interaction promoted the expression of cps genes and ultimately led to an increase in CPS production.
Discussion
Streptococcus pneumoniae is a major pathogen causing pneumonia, otitis media, meningitis and sepsis globally, leading to over 500,000 childhood fatalities each year (Briles et al., 2019). CPS is the most crucial virulence factors. During pathogenesis, S. pneumoniae adapts to various host ecosystems by fine-tuning the transcript levels of its cps genes and controlling CPS production. Many carbon sources exist in the host ecological niche, such as the nasopharynx, which is predominantly rich in galactose, N-acetylglucosamine (GlcNAc) and mannose, or blood, which is dominated by glucose (Aprianto et al., 2018; Hobbs et al., 2018). Recent report indicates that various exogenous carbohydrates can affect CPS production and gene expression (Werren et al., 2023). For example, in glucose-enriched blood, the increased transcript levels of the cps gene promote CPS synthesis (Kim et al., 1999). This may be an important reason why diabetes is considered an independent risk factor for severe S. pneumoniae infection (Ishiguro, 2016). However, the specific factors involved in this regulation remain unclear.
CcpA is a major regulator of carbohydrate metabolism. Previous studies have shown that in Streptococcus suis, CcpA deficiency can reduce CPS synthesis (Willenborg et al., 2011), and ΔccpA mutant strains of group A streptococci have significantly reduced virulence in invasive mouse models (Sonenshein, 2007). However, the specific mechanism of CcpA on CPS synthesis in S. pneumoniae serotype 2 strains D39 has not been reported. In this study, we elucidate the pivotal role of CcpA in the regulation of carbohydrate metabolism and CPS.
We discovered that CcpA promotes CPS synthesis in an environment where glucose is the sole carbon source, indicating that CcpA may act as a transcriptional regulator of the cps operons. To investigate this, we first analyzed the cps promoter sequence and identified a conserved CcpA binding motif. We then experimentally confirmed that CcpA specifically binds to the cps promoter, facilitating the transcription of cps loci and thereby promoting CPS synthesis. These findings demonstrated that CcpA positively regulated CPS synthesis in glucose as the sole carbon source.
We observed that glucose positively regulates CPS synthesis in wild-type strains, whereas the effect disappeared after ccpA deficiency, suggesting that CcpA may be involved in the regulation. Further findings revealed that high glucose promoted the transcriptional activity of the cps promoter, but this effect was eliminated after CcpA deletion. These evidences suggested that the regulatory effect of glucose should be realized through the transcriptional regulation of the cps locus by CcpA. Interestingly, we found that neither the level of CcpA protein expression nor its ability to bind to the cps promoter was affected by glucose concentration, which was consistent with the results reported in Bacillus subtilis (Mijakovic et al., 2005), implying that alternative regulatory mechanisms are likely involved in glucose regulation of CPS synthesis.
In many bacteria, CcpA functions as a regulatory protein by forming a complex with HPr-Ser46~P, enabling specific binding to cre sequences. In Bacillus subtilis, the interaction between CcpA and HPr-Ser46~P enables CcpA to specifically recognize gnt cis-acting catabolic response elements (Fujita et al., 1995). Additionally, the activation of ilv-leu catabolism metabolites also requires the presence of CcpA and the HPr-Ser46~P complex (Fujita et al., 2014). In Streptococcus suis, the protein HP0197 promotes the phosphorylation level of phosphocarrier protein HPr at residues Ser-46, which enhances the binding of CcpA to cps promoter and thus regulates carbohydrate utilization and CPS synthesis gene expression (Willenborg et al., 2011). Therefore, we speculated that HPr-Ser46~P regulated CPS synthesis by promoting the binding of CcpA to the cps promoter. This hypothesis was confirmed by EMSA experiments. Compared with unmodified HPr protein, HPr-Ser46~P protein significantly promoted the binding of CcpA protein to cps promoters. Meanwhile, it was also observed that the form of HPr-Ser46~P protein increased significantly with the increase of glucose concentration, suggesting that glucose promoted CPS synthesis by promoting the phosphorylation of HPr-Ser46.
Studies have shown that HPr has two phosphorylation sites in low G + C Gram-positive bacteria: His-15 site and Ser-46 site. It has been reported that the HPr-His15~P protein form of Bacillus subtilis cannot bind to CcpA (Reizer et al., 1996). But another study has shown that HPr-His15~P in Bacillus subtilis may exerts its regulatory effect by stimulating the binding of CcpA and cps promoter through FBP and Glc-6-P (Horstmann et al., 2007). Our results shown that the form of HPr-His15~P protein also increases significantly when glucose concentration increases (Fig. 6A), suggesting that glucose may also promote capsule synthesis by promoting the phosphorylation of His15. Further research is needed to determine whether it has the same regulatory mechanism as Bacillus subtilis.
Based on the existing experimental evidence, we elucidated the hypothesis that glucose regulates CPS synthesis via CcpA in the presence of glucose. It promotes the formation of HPr-Ser46~P, which synergistically interacts with CcpA to promote CPS synthesis by facilitating the binding of CcpA to the cps promoter. These findings in this study reveal a novel mechanism for the regulation of CPS synthesis by carbon metabolism, which provides a new target for the prevention and treatment of S. pneumoniae.
Acknowledgments
This study was supported by Hunan Provincial Natural Science Foundation of China (2025JJ80470).
Conflict of Interest
The authors have declared that no competing interest exists.
Ethical Statements
The ARRIVE guidelines were followed during study involving laboratory animals. All animal experiments were performed in accordance with the regulation for animal experimentation of Chongqing Province, China, and were permitted by the Ethics Committee of Chongqing Medical University (IACUC-CQMU-2023-03001).
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.71150/jm.2411024.
Fig. S2.
Deletion of ptsH reduces virulence. (A) Survival of mice pneumonia model infected with D39s, D39ΔptsH, and D39ΔptsH::ptsH (bacterial load of 108 CFU) (n = 10). (B, C, D, and E) Nasal lavage, lung homogenate, spleen homogenate, and cardiac blood bacterial load in mice infected with different S.pn strains D39s, D39ΔptsH, and D39ΔptsH::ptsH (bacterial load of 2 × 107 CFU) for 48h (n = 5). Survival was analyzed using log-rank tests. ***, P < 0.001.
jm-2411024-Supplementary-Fig-S2.pdf
Fig. 1.CcpA promotes CPS synthesis in an environment with glucose as the sole carbon source.
(A) Western blot to determine the CPS content of D39s, D39ΔccpA and D39ΔccpA::ccpA. (B) Uronic acid assay to determine the CPS content of D39s, D39ΔccpA and D39ΔccpA::ccpA. (C) Macrophage adhesion capacity of D39s, D39ΔccpA and D39ΔccpA::ccpA. (D) Macrophage antiphagocytic capacity of D39s, D39ΔccpA and D39ΔccpA::ccpA. Data are shown as mean ± SD of three independent experiments (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
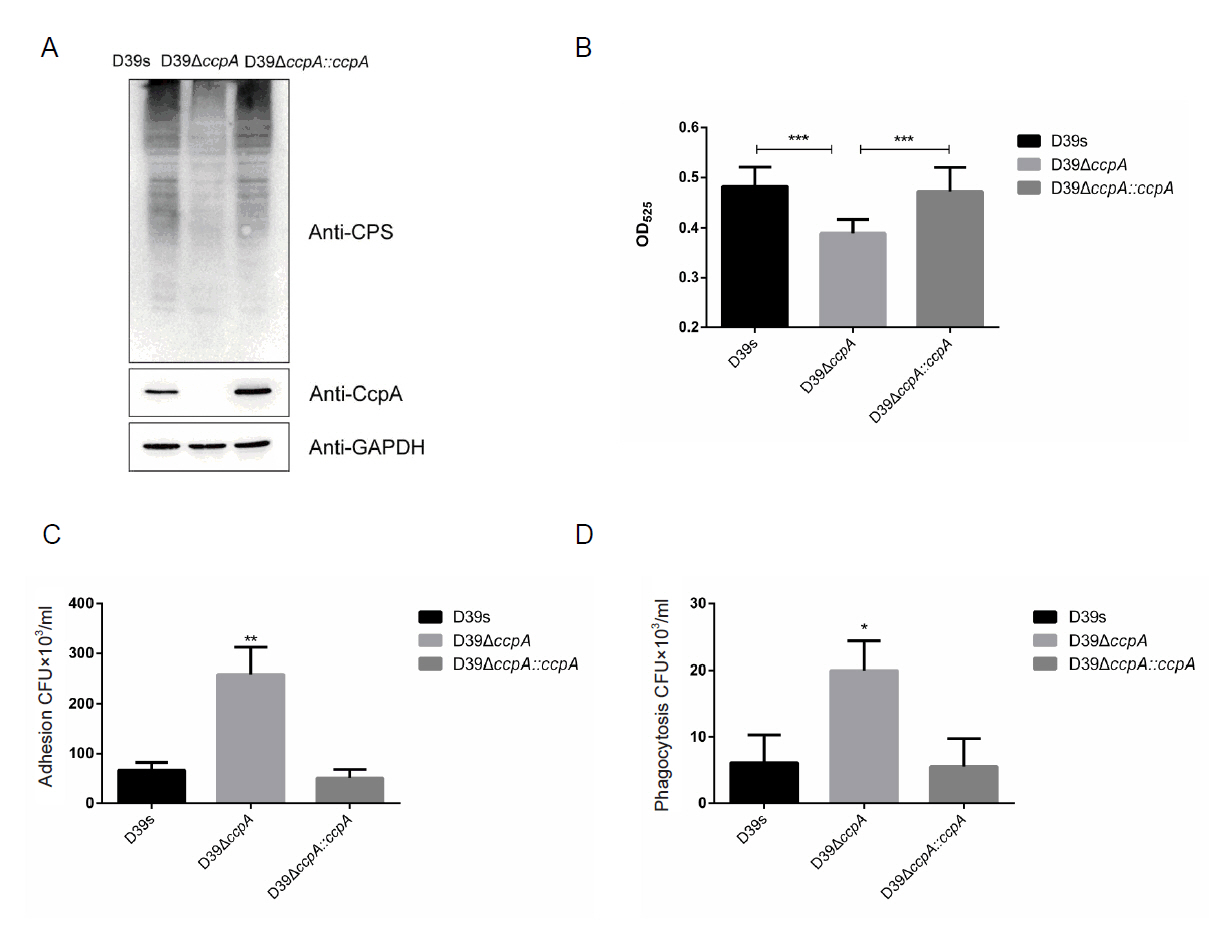
Fig. 2.CcpA binds specifically to the cps promoter to promote cps operon transcription. (A) Typical CcpA binding sites were compared to cps promoter sequences using the MEME online site. Typical cre sequences and predicted cre sequences on the cps promoter are shown in the figure, with N representing any base and W representing A or T. (B) EMSA verified the specific binding of CcpA to cps promoter. (C) Real-time fluorescence quantitative PCR to detect the transcript levels of genes related to CPS synthesis. (D) Luciferase reporter strains D39s-Pcps-luc and ΔccpA-Pcps-luc were assayed for cps promoter activity. Data are shown as mean ± SD of three independent experiments (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
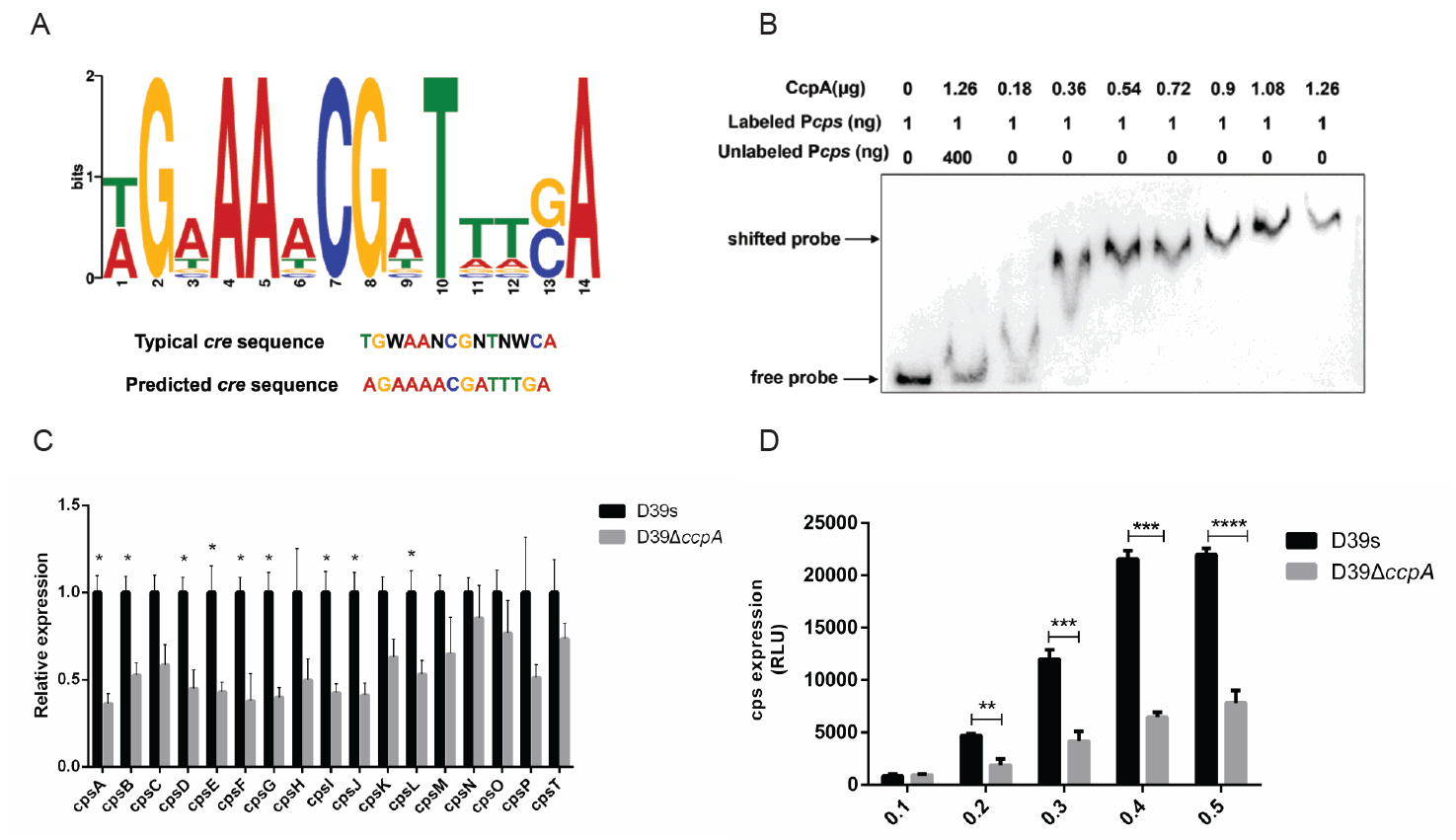
Fig. 3.CcpA is involved in the regulation of glucose on capsule synthesis. (A) Western blot assay to determine the CPS expression of D39s and D39ΔccpA at different glucose concentrations. (B) Real-time fluorescence quantitative PCR to detect the transcript levels of CPS synthesis-related genes in D39s, D39ΔccpA and D39ΔptsH at different glucose concentrations. (C) Luciferase reporter strains D39s-Pcps-luc and ΔccpA-Pcps-luc were assayed for cps promoter activity at different glucose concentrations. (D) Real-time fluorescence quantitative PCR to detect the transcript levels of ccpA in D39s and D39ΔccpA at different glucose concentrations. (E) EMSA detected the binding of CcpA to cps promoter at different glucose concentrations. Data are shown as mean ± SEM of three independent experiments (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
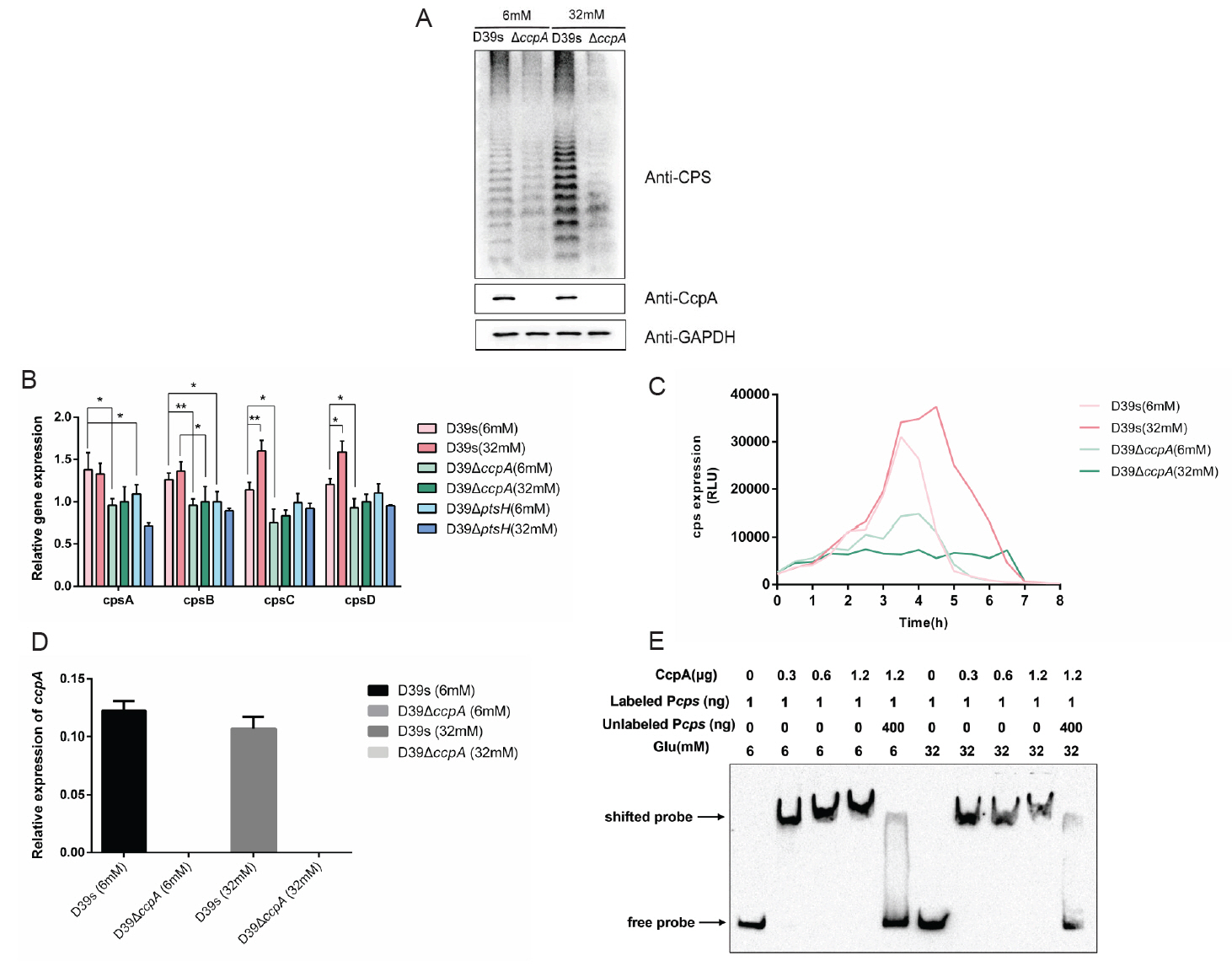
Fig. 4.HPr regulates capsule synthesis through the cps locus. (A) Real-time fluorescence quantitative PCR to detect the transcript levels of ptsH in D39s, D39ΔptsH and D39ΔptsH::ptsH. (B) Western blot assay to determine the HPr protein expression level and CPS expression of D39s, D39ΔptsH and D39ΔptsH::ptsH. (C) Uronic acid assay to determine the CPS content in D39s, D39ΔptsH and D39ΔptsH::ptsH. (D) Real-time fluorescence quantitative PCR to detect the transcript levels of CPS synthesis-related genes in D39s, D39ΔptsH and D39ΔptsH::ptsH. (E) The luciferase reporter strains D39s-Pcps-luc and D39ΔptsH-Pcps-luc were assayed for cps promoter activity. Data are shown as mean ± SEM of three independent experiments (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
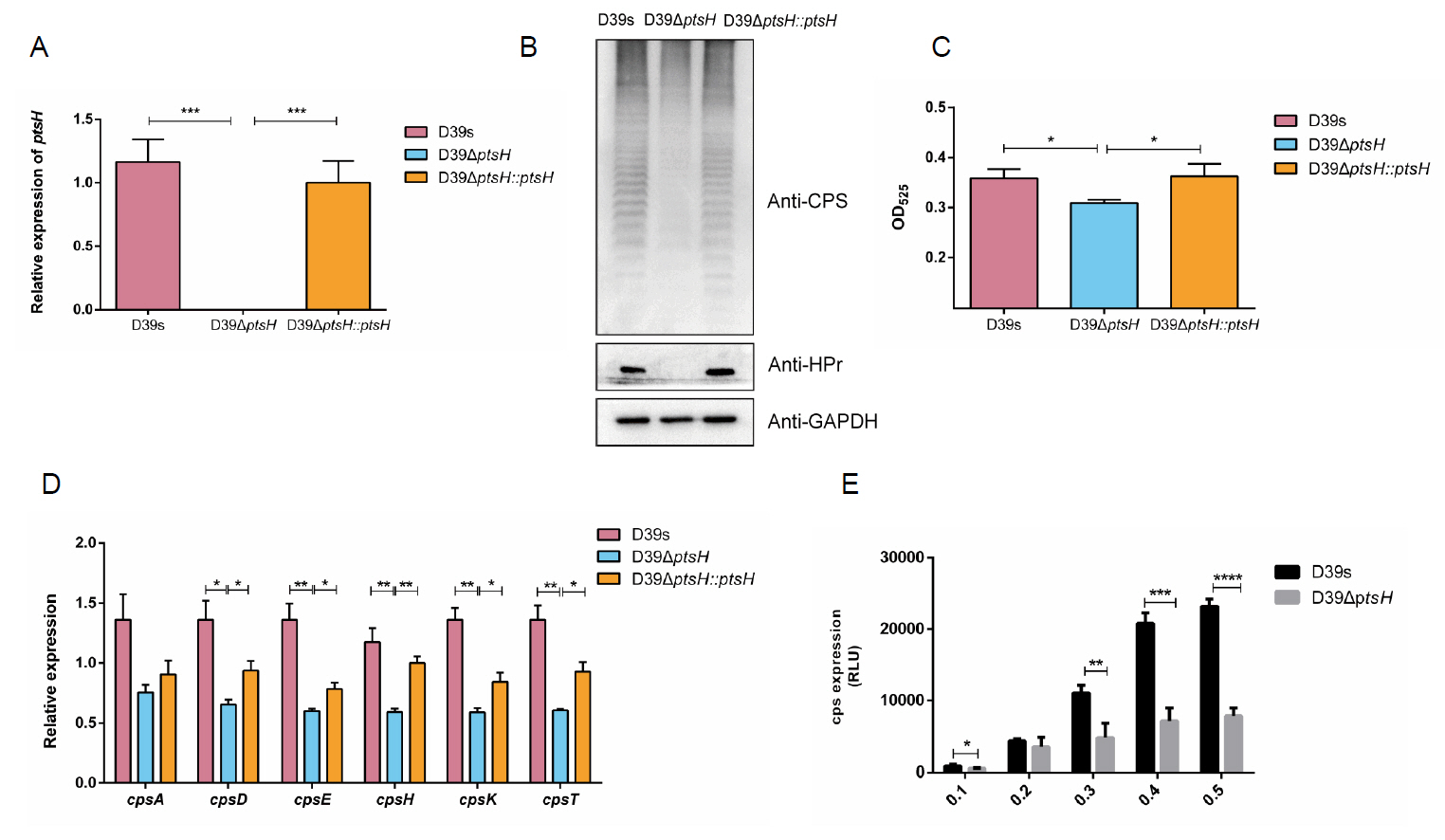
Fig. 5.HPr is involved in the regulation of glucose on capsule synthesis. (A) Western blot assay to determine the CPS expression of D39s and D39ΔptsH at different glucose concentrations. (B) Uronic acid assay to determine the CPS content of D39s and D39ΔptsH at different glucose concentrations. (C) Luciferase reporter strains D39s-Pcps-luc and D39ΔptsH Pcps-luc were assayed for cps promoter activity at different glucose concentrations. Data are shown as mean ± SEM of three independent experiments (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
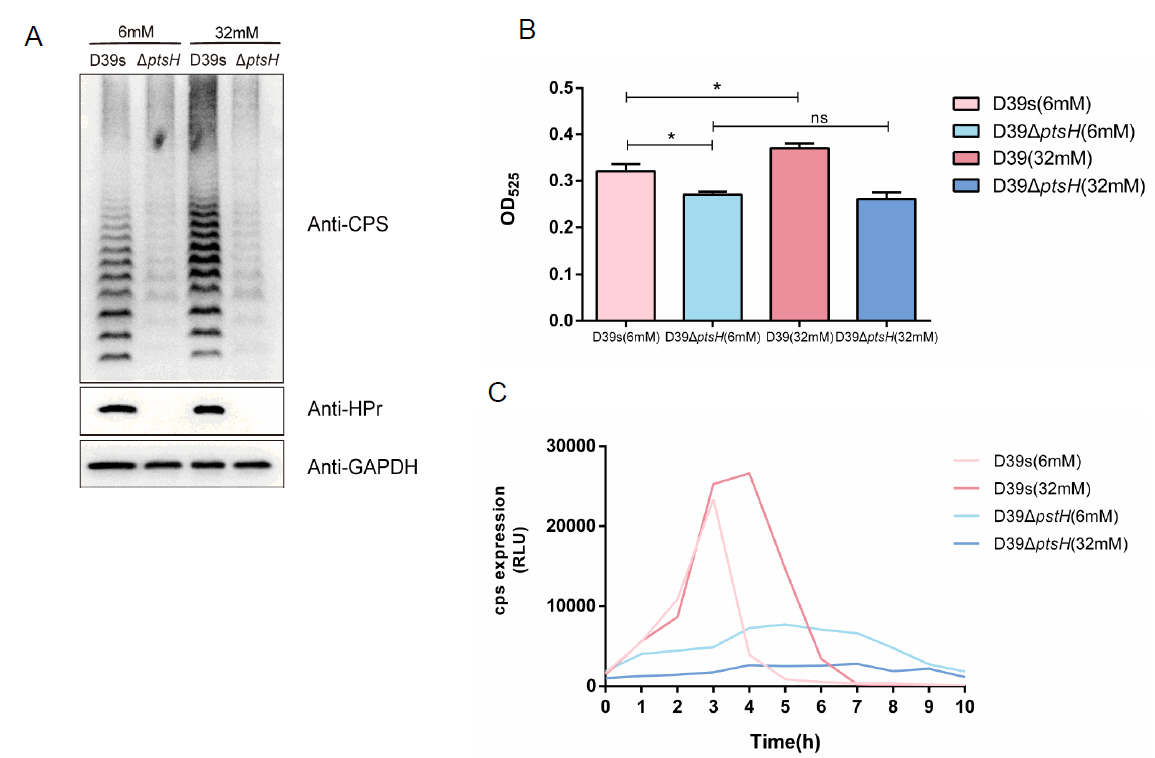
Fig. 6.Glucose promotes the formation of HPr-Ser46~P to promoting the regulation of CcpA on the capsule. (A) Non-denaturing gel analysis of the HPr and its phosphorylated forms (HPr-His15~P or HPr-Ser46~P) to determine the amount of HPr-Ser46~P at different glucose concentrations in WT strains. The ptsH mutant as negative control, HPr S46D strain as positive control (Equal amounts of cell extracts untreated [−] or incubated at 70°C for 10 min [+] to hydrolyze the heat-labile HPr-His15~P). (B) Western blot assay to determine the expression of CPS in HPr S46D mutant bacteria at 6mM glucose concentrations. (C) EMSA to detect the effect of the addition of HPr and HPr-Ser46~P proteins on the binding of CcpA to cps promoter.
References
- Aprianto R, Slager J, Holsappel S, Veening JW. 2018. High-resolution analysis of the pneumococcal transcriptome under a wide range of infection-relevant conditions. Nucleic Acids Res. 46: 9990–10006. ArticlePubMedPMC
- Briles DE, Paton JC, Mukerji R, Swiatlo E, Crain MJ. 2019. Pneumococcal vaccines. Microbiol Spectr. 7: 20.ArticlePubMedPMCPDF
- Butters C, Phuong LK, Cole T, Gwee A. 2019. Prevalence of immunodeficiency in children with invasive pneumococcal disease in the pneumococcal vaccine era: A systematic review. JAMA Pediatr. 173: 1084–1094. ArticlePubMed
- Chauvaux S. 1996. CcpA and HPr(ser-P): mediators of catabolite repression in Bacillus subtilis. Res Microbiol. 147: 518–522.ArticlePubMed
- Dao TH, Rosch JW. 2021. JMM profile: Streptococcus pneumoniae : sugar-coated captain of the men of death. J Med Microbiol. 70: 001446.ArticlePubMedPMC
- de Vos AF, Dessing MC, Lammers AJJ, Florquin S, de Boer OJ, et al. 2015. The polysaccharide capsule of Streptococcus pneumoniae partially impedes MyD88-mediated immunity during pneumonia in mice. PLoS One. 10: e0118181.ArticlePubMedPMC
- Deutscher J, Herro R, Bourand A, Mijakovic I, Poncet S. 2005. P-Ser-HPr—a link between carbon metabolism and the virulence of some pathogenic bacteria. Biochim Biophys Acta Proteins Proteomics. 1754: 118–125. Article
- Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. 1995. Protein kinase‐dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram‐positive bacteria. Mol Microbiol. 15: 1049–1053. ArticlePubMed
- Fleming E, Lazinski DW, Camilli A. 2015. Carbon catabolite repression by seryl phosphorylated HPr is essential to Streptococcus pneumoniae in carbohydrate‐rich environments. Mol Microbiol. 97: 360–380. ArticlePubMedPMC
- Fujita Y. 2009. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci Biotechnol Biochem. 73: 245–259. ArticlePubMed
- Fujita Y, Miwa Y, Galinier A, Deutscher J. 1995. Specific recognition of the Bacillus subtilis gnt cis‐acting catabolite‐responsive element by a protein complex formed between CcpA and seryl‐phosphorylated HPr. Mol Microbiol. 17: 953–960. ArticlePubMed
- Fujita Y, Satomura T, Tojo S, Hirooka K. 2014. CcpA-mediated catabolite activation of the Bacillus subtilis ilv-leu operon and its negation by either CodY- or TnrA-mediated negative regulation. J Bacteriol. 196: 3793–3806. ArticlePubMedPMCPDF
- Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, et al. 2015. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 28: 871–899. ArticlePubMedPMCPDF
- Geno KA, Hauser JR, Gupta K, Yother J. 2014. Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability. J Bacteriol. 196: 1992–2003. ArticlePubMedPMC
- Giammarinaro P, Paton JC. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun. 70: 5454–5461. ArticlePubMedPMCPDF
- Görke B, Stülke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 6: 613–624. ArticlePubMedPDF
- Henkin TM. 1996. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol Lett. 135: 9–15. ArticlePubMed
- Hobbs JK, Pluvinage B, Boraston AB. 2018. Glycan‐metabolizing enzymes in microbe-host interactions: The Streptococcus pneumoniae paradigm. FEBS Lett. 592: 3865–3897. ArticlePubMedPDF
- Horstmann N, Seidel G, Aung-Hilbrich LM, Hillen W. 2007. Residues His-15 and Arg-17 of HPr participate differently in catabolite signal processing via CcpA. J Biol Chem. 282: 1175–1182. ArticlePubMed
- Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 78: 704–715. ArticlePubMedPMCPDF
- Ishiguro T, Kagiyama N, Uozumi R, Odashima K, Kurashima K, et al. 2016. Risk factors for the severity and mortality of pneumococcal pneumonia: importance of premorbid patients' performance status. J Infect Chemother. 22: 685–691. ArticlePubMed
- Kim JO, Romero-Steiner S, Sørensen UBS, Blom J, Carvalho M, et al. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 67: 2327–2333. ArticlePubMedPMCPDF
- Mijakovic I, Petranovic D, Bottini N, Deutscher J, Ruhdal Jensen P. 2005. Protein-tyrosine phosphorylation in Bacillus subtilis. J Mol Microbiol Biotechnol. 9: 189–197. ArticlePubMed
- Moscoso M, Garcia E. 2009. Transcriptional regulation of the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae : a bioinformatic analysis. DNA Res. 16: 177–186. ArticlePubMedPMC
- Reizer J, Bergstedt U, Galinier A, Küster E, Saier MH, et al. 1996. Catabolite repression resistance of gnt operon expression in Bacillus subtilis conferred by mutation of His-15, the site of phosphoenolpyruvate-dependent phosphorylation of the phosphocarrier protein HPr. J Bacteriol. 178: 5480–5486. ArticlePubMedPMCPDF
- Roy DJ, Casabon I, Vaillancourt K, Huot JL, Vadeboncoeur C. 2008. Streptococci and lactococci synthesize large amounts of HPr(Ser-P)(His~P). Can J Microbiol. 54: 941–949. ArticlePubMed
- Shainheit MG, Mulé M, Camilli A. 2014. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun. 82: 694–705. ArticlePubMedPMCPDF
- Shelburne SA 3rd, Keith D, Horstmann N, Sumby P, Davenport MT, et al. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci USA. 105: 1698–1703. ArticlePubMedPMC
- Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol. 5: 917–927. ArticlePubMedPDF
- Su T, Nakamoto R, Chun YY, Chua WZ, Chen JH, et al. 2021. Decoding capsule synthesis in Streptococcus pneumoniae. FEMS Microbiol Rev. 45: fuaa067.ArticlePubMedPDF
- Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 67: 5190–5196. ArticlePubMedPMCPDF
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, et al. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 18: 1191–1210. PubMedPMC
- Troxler LJ, Werren JP, Schaffner TO, Mostacci N, Vermathen P, et al. 2019. Carbon source regulates polysaccharide capsule biosynthesis in Streptococcus pneumoniae. J Biol Chem. 294: 17224–17238. ArticlePubMedPMC
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, et al. 2018. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15. Lancet Glob Health. 6: e744–e757. ArticlePubMedPMC
- Warner JB, Lolkema JS. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol Mol Biol Rev. 67: 475–490. ArticlePubMedPMCPDF
- Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, et al. 2007. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 9: 1162–1171. ArticlePubMed
- Weickert MJ, Chambliss GH. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 87: 6238–6242. ArticlePubMedPMC
- Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet. 378: 1962–1973. ArticlePubMedPMC
- Wen Z, Sertil O, Cheng Y, Zhang S, Liu X, et al. 2015. Sequence elements upstream of the core promoter are necessary for full transcription of the capsule gene operon in Streptococcus pneumoniae strain D39. Infect Immun. 83: 1957–1972. ArticlePubMedPMCPDF
- Werren JP, Mostacci N, Gjuroski I, Holivololona L, Troxler LJ, et al. 2023. Carbon source-dependent capsule thickness regulation in Streptococcus pneumoniae. Front Cell Infect Microbiol. 13: 1279119.ArticlePubMedPMC
- Willenborg J, Fulde M, De Greeff A, Rohde M, Smith HE, et al. 2011. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology. 157: 1823–1833. ArticlePubMed
- Wu K, Xu H, Zheng Y, Wang L, Zhang X, et al. 2016. CpsR, a GntR family regulator, transcriptionally regulates capsular polysaccharide biosynthesis and governs bacterial virulence in Streptococcus pneumoniae. Sci Rep. 6: 29255.ArticlePubMedPMCPDF
- Yang Y, Zhang L, Huang H, Yang C, Yang S, et al. 2017. A flexible binding site architecture provides new insights into CcpA global regulation in Gram-positive bacteria. mBio. 8: e02004–16. ArticlePubMedPMCPDF
- Yother J. 2011. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol. 65: 563–581. ArticlePubMed
- Zhang Y, Zhang J, Xiao J, Wang H, Yang R, et al. 2023. comCDE (competence) operon is regulated by CcpA in Streptococcus pneumoniae D39. Microbiol Spectrum. 11: e00012–23.ArticlePubMedPMC
- Zheng Y, Zhang X, Wang X, Wang L, Zhang J, et al. 2017. ComE, an essential response regulator, negatively regulates the expression of the capsular polysaccharide locus and attenuates the bacterial virulence in Streptococcus pneumoniae. Front Microbiol. 8: 277.ArticlePubMedPMC
- Zhou X, Liu J, Zhang Z, Cui B, Wang Y, et al. 2022. Characterization of Streptococcus pneumoniae macrolide resistance and its mechanism in Northeast China over a 20-year period. Microbiol Spectr. 10: e00546–22. ArticlePubMedPMCPDF
Citations
Citations to this article as recorded by

- The metabolic, microbial and immunological demands of pneumococcal colonisation
Daniel R. Neill, Thomas B. Clarke, N.Luisa Hiller
PLOS Pathogens.2025; 21(11): e1013675. CrossRef








 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article







