Articles
- Page Path
- HOME > J. Microbiol > Volume 63(6); 2025 > Article
-
Review
Targeting innate immune sensors for therapeutic strategies in infectious diseases - Seyun Shin1, Young Ki Choi2,*, SangJoon Lee1,3,*
-
Journal of Microbiology 2025;63(6):e2503009.
DOI: https://doi.org/10.71150/jm.2503009
Published online: June 30, 2025
1Department of Biological Science, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea
2Center of Study of Emerging and Re-emerging Viruses, Korea Virus Research Institute, Institute for Basic Science (IBS), Daejeon 34126, Republic of Korea
3Graduate School of Health Science and Technology, Ulsan National institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea
- *Correspondence Young Ki Choi choiki55@ibs.re.kr SangJoon Lee sangjoon.lee@unist.ac.kr
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- Introduction
- Toll-Like Receptors (TLRs)
- Nod-Like Receptors
- RIG-I-Like Receptors (RLRs)
- Other Cytosolic Innate Immune Sensor
- Importance of Cytosolic Innate Immune Sensors in Infectious, Inflammatory, and Metabolic Diseases
- Virus Targeting Therapeutic Strategies
- Host Targeting Therapeutic Strategies
- Conclusion
- Notes
- References
ABSTRACT
- The innate immune system relies on innate immune sensors, such as pattern recognition receptors (PRRs), to detect pathogens and initiate immune responses, crucial for controlling infections but also implicated in inflammatory diseases. These innate immune sensors, including Toll-like receptors (TLRs), nod-like receptors (NLRs), RIG-I-like receptors (RLRs), absent in melanoma 2 (AIM2), and Z-DNA binding protein 1 (ZBP1) trigger signaling pathways that produce cytokines, modulating inflammation and cell death. Traditional therapies focus on directly targeting pathogens; however, host-targeting therapeutic strategies have emerged as innovative approaches to modulate innate immune sensor activity. These strategies aim to fine-tune the immune response, either enhancing antiviral defenses or mitigating hyperinflammation to prevent tissue damage. This review explores innate immune sensor-based therapeutic approaches, including inhibitors, agonists, and antagonists, that enhance antiviral defense or suppress harmful inflammation, highlighting innate immune sensors as promising targets in infectious and inflammatory disease treatment.
Introduction
Toll-Like Receptors (TLRs)
Nod-Like Receptors
RIG-I-Like Receptors (RLRs)
Other Cytosolic Innate Immune Sensor
Importance of Cytosolic Innate Immune Sensors in Infectious, Inflammatory, and Metabolic Diseases
Virus Targeting Therapeutic Strategies
Host Targeting Therapeutic Strategies
Conclusion
Acknowledgments
We apologize to our colleagues in this field whose work could not be cited owing to space limitations. We thank the members of the Lee Laboratory (Viral Immunology Laboratory) for their helpful comments and suggestions. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1C1C1007544, 2024M3A9H5043152 to S.L.), a grant from the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea, under the Korea Health Technology R&D Project (RS-2022-KH128422(HV22C015600) to S.L.), and the Institute for Basic Science (IBS), Republic of Korea (IBS-R801-D1 to Y.K.C, IBS-R801-D9-A09, IBS-R801-D1-2025-a02 to S.L.). Moreover, this work was supported by The Circle Foundation (Republic of Korea) through the selection of the UNIST Pandemic Treatment Research Center as the 2023 The Circle Foundation Innovative Science Technology Center (2023 TCF Innovative Science Project-01 to S.L.). Additionally, this study received funding from the Republic of Korea’s National Institute of Health (#2025ER160200, #2025ER240100 to S.L.). Additional support was provided by research funds from Ulsan National Institute of Science & Technology (UNIST) (1.220112.01, 1.220107.01 to S.L.), The Korean Society of Ginseng 2023 (S.L.), and a grant from Yuhan Corporation (S.L.).
Conflict of Interest
The authors have no conflict of interest.
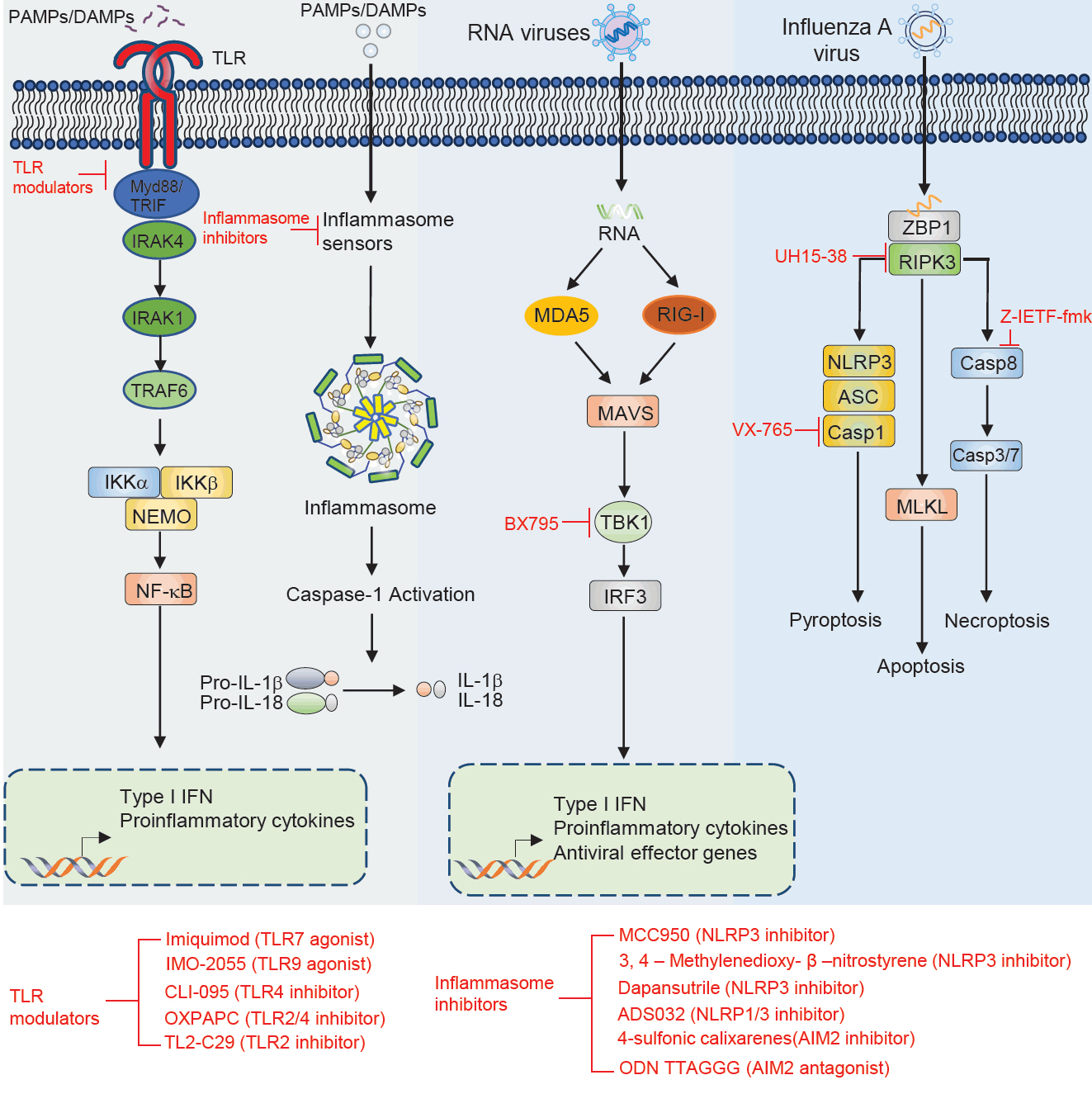
| Therapeutic molecule | Target | Disease | Effect | Outcome | Reference |
|---|---|---|---|---|---|
| Imiquimod | TLR7 | Tumor | Agonist | Induce the production of cytokine | Hemmi et al. (2002), Wang et al. (2005) |
| IMO-2055 | TLR9 | Tumor | Agonist | Enhance antitumor efficacy | Smith et al. (2014) |
| CLI-095 | TLR4 | Atherosclerosis | Inhibitor | Suppress LPS induced inflammation | Alibashe-Ahmed et al. (2019), Kawamoto et al. (2008), Wang et al. (2016) |
| OXPAPC | TLR2, TLR4 | Sepsis shock | Inhibitor | Inhibits non-canonical pyroptosis | Chu et al. (2018) |
| TL2-C29 | TLR2 | Hepatitis C virus | Inhibitor | Inhibitor of TLR2/1 signaling | Mistry et al. (2015), Oliveira-Nascimento et al. (2012) |
| MCC950 | NLRP3 | Inflammatory diseases (atherosclerosis, myocardial fibrosis, spinal cord injury, neurological disorders, intestinal inflammation) | Inhibitor | Alleviates symptoms of associated inflammatory conditions | Coll et al. (2022), Dempsey et al. (2017), Gao et al. (2019), Jiao et al. (2020b), Zeng et al. (2021) |
| 3,4-Methylenedioxy-β-nitrostyrene | NLRP3 | Renal ischemia | Inhibitor | Protects from renal ischemia | Uysal et al. (2022) |
| Dapansutrile | NLRP3 | Autoimmune encephalomyelitis, acute arthritis | Inhibitor | Atternuates clinical signs and improves prognosis | Klück et al. (2020), Marchetti et al. (2018a, 2018b), Sánchez-Fernández et al. (2019) |
| ADS032 | NLRP1, NLRP3 | IAV-induced pulmonary inflammation and disease severity | Inhibitor | reduces acute silicosis-associated pulmonary inflammation | Docherty et al. (2023) |
| 4-Sulfonic calixarenes | AIM2 | Post-stroke immunosuppression | Inhibitor | AIM2-dependent post-stroke T cell death inhibition | Green et al. (2023) |
| ODN TTAGGG | AIM2 | MCMV and L. monocytogenes | Antagonist | Blocks AIM2 inflammasome activation in response to cytosolic dsDNA | Eichholz et al. (2016), Kaminski et al. (2013) |
| UH15-38 | RIPK3 | Blocked IAV-triggered necroptosis in alveolar epithelial cells in vivo | Inhibitor | UH15-38 ameliorated lung inflammation and prevented mortality | Gautam et al. (2024) |
| z-IETF-fmk | Caspase8 | Lethal bacterial peritonitis and pneumonia | Inhibitor | z-IETD-fmk induces pro-inflammatory cytokine productin in neutrophils but not in macrophages | Lentini et al. (2023) |
| VX-765 | Caspase1 | CNS disease | Inhibitor | Reduces CNS inflammation, prevents axonal injury, improves neurobehavioral in EAE | McKenzie et al. (2018) |
- Alibashe-Ahmed M, Brioudes E, Reith W, Bosco D, Berney T. 2019. Toll-like receptor 4 inhibition prevents autoimmune diabetes in NOD mice. Sci Rep. 9: 19350.ArticlePubMedPMCPDF
- Almeida-da-Silva CLC, Savio LEB, Coutinho-Silva R, Ojcius DM. 2023. The role of NOD-like receptors in innate immunity. Front Immunol. 14: 1122586.ArticlePubMedPMC
- Badia R, Garcia-Vidal E, Ballana E. 2022. Viral-host dependency factors as therapeutic targets to overcome antiviral drug-resistance: A focus on innate immune modulation. Front Virol. 2: 935933.Article
- Bakheit AH, Darwish H, Darwish IA, Al-Ghusn AI. 2023. Remdesivir. Profiles Drug Subst Excip Relat Methodol. 48: 71–108. ArticlePubMedPMC
- Balachandran Y, Caldwell S, Aulakh GK, Singh B. 2022. Regulation of TLR10 expression and its role in chemotaxis of human neutrophils. J Innate Immun. 14: 629–642. ArticlePubMedPMCPDF
- Baldo BA. 2014. Side effects of cytokines approved for therapy. Drug Saf. 37: 921–943. ArticlePubMedPMCPDF
- Broz P, Dixit VM. 2016. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat Rev Immunol. 16: 407–420. ArticlePubMedPDF
- Bruns AM, Leser GP, Lamb RA, Horvath CM. 2014. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol Cell. 55: 771–781. ArticlePubMedPMC
- Chang TH, Liao CL, Lin YL. 2006. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-κB activation. Microbes Infect. 8: 157–171. ArticlePubMed
- Chattopadhyay S, Sen GC. 2014. dsRNA-activation of TLR3 and RLR signaling: Gene induction-dependent and independent effects. J Interferon Cytokine Res. 34: 427–436. ArticlePubMedPMC
- Chavarria-Smith J, Vance RE. 2013. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 9: e1003452. ArticlePubMedPMC
- Chiang JJ, Davis ME, Gack MU. 2014. Regulation of RIG-I-like receptor signaling by host and viral proteins. Cytokine Growth Factor Rev. 25: 491–505. ArticlePubMedPMC
- Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, et al. 2018. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat Commun. 9: 996.ArticlePubMedPMCPDF
- Chuenchor W, Jin T, Ravilious G, Xiao TS. 2014. Structures of pattern recognition receptors reveal molecular mechanisms of autoinhibition, ligand recognition and oligomerization. Curr Opin Immunol. 26: 14–20. ArticlePubMed
- Coll RC, Schroder K, Pelegrin P. 2022. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 43: 653–668. ArticlePubMed
- Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, et al. 2017. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 61: 306–316. ArticlePubMed
- Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, et al. 2015. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci USA. 112: 4050–4055. ArticlePubMedPMC
- Devos M, Tanghe G, Gilbert B, Dierick E, Verheirstraeten M, et al. 2020. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J Exp Med. 217: e20200678. ArticlePubMedPMC
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303: 1529–1531. ArticlePubMed
- Docherty CAH, Fernando AJ, Rosli S, Lam M, Dolle RE, et al. 2023. A novel dual NLRP1 and NLRP3 inflammasome inhibitor for the treatment of inflammatory diseases. Clin Transl Immunol. 12: e1455. Article
- Duncan JA, Canna SW. 2018. The NLRC4 inflammasome. Immunol Rev. 281: 115–123. ArticlePubMedPMC
- Eaves-Pyles T, Murthy K, Liaudet L, Virag L, Ross G, et al. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 166: 1248–1260. ArticlePubMedPDF
- Eichholz K, Bru T, Tran TT, Fernandes P, Welles H, et al. 2016. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog. 12: e1005871. ArticlePubMedPMC
- Enzan N, Matsushima S, Ikeda S, Okabe K, Ishikita A, et al. 2023. ZBP1 protects against mtDNA-induced myocardial inflammation in failing hearts. Circ Res. 132: 1110–1126. ArticlePubMedPMC
- Fang R, Jiang Q, Zhou X, Wang C, Guan Y, et al. 2017. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 13: e1006720. ArticlePubMedPMC
- Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, et al. 2008. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 83: 692–701. ArticlePubMedPDF
- Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, et al. 2007. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr Biol. 17: 1140–1145. ArticlePubMed
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 458: 509–513. ArticlePubMedPMCPDF
- Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, et al. 2021. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 592: 296–301. ArticlePubMedPMCPDF
- Fitzgerald KA, Kagan JC. 2020. Toll-like receptors and the control of immunity. Cell. 180: 1044–1066. ArticlePubMedPMC
- Franchi L, Warner N, Viani K, Nunez G. 2009. Function of NOD-like receptors in microbial recognition and host defense. Immunol Rev. 227: 106–128. ArticlePubMedPMC
- Frew BC, Joag VR, Mogridge J. 2012. Proteolytic processing of NLRP1B is required for inflammasome activity. PLoS Pathog. 8: e1002659. ArticlePubMedPMC
- Fukuda K, Okamura K, Riding RL, Fan X, Afshari K, et al. 2021. AIM2 regulates anti-tumor immunity and is a viable therapeutic target for melanoma. J Exp Med. 218: e20201138. ArticlePubMedPMCPDF
- Gao R, Shi H, Chang S, Gao Y, Li X, et al. 2019. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 74: 105575.ArticlePubMed
- Gautam A, Boyd DF, Nikhar S, Zhang T, Siokas I, et al. 2024. Necroptosis blockade prevents lung injury in severe influenza. Nature. 628: 835–843. ArticlePubMedPMCPDF
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. 2001. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 167: 1882–1885. ArticlePubMedPDF
- Ghimire L, Paudel S, Jin L, Jeyaseelan S. 2020. The NLRP6 inflammasome in health and disease. Mucosal Immunol. 13: 388–398. ArticlePubMedPMCPDF
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, et al. 2006. Essential role of MDA-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 103: 8459–8464. ArticlePubMedPMC
- Gong T, Liu L, Jiang W, Zhou R. 2020. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 20: 95–112. ArticlePubMedPDF
- Goubau D, Deddouche S, Reis e Sousa C. 2013. Cytosolic sensing of viruses. Immunity. 38: 855–869. ArticlePubMedPMC
- Green JP, El-Sharkawy LY, Roth S, Zhu J, Cao J, et al. 2023. Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome. iScience. 26: 106758.ArticlePubMedPMC
- Grundeis F, Ansems K, Dahms K, Thieme V, Metzendorf MI, et al. 2023. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 1: CD014962.ArticlePubMed
- Hafler DA. 2007. Cytokines and interventional immunology. Nat Rev Immunol. 7: 423–424. ArticlePDF
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410: 1099–1103. ArticlePubMedPDF
- Hellmich KA, Levinsohn JL, Fattah R, Newman ZL, Maier N, et al. 2012. Anthrax lethal factor cleaves mouse NLRP1B in both toxin-sensitive and toxin-resistant macrophages. PLoS One. 7: e49741. ArticlePubMedPMC
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, et al. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 3: 196–200. ArticlePubMedPDF
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408: 740–745. ArticlePubMedPDF
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, et al. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 458: 514–518. ArticlePubMedPMCPDF
- Hsu A, Granneman GR, Bertz RJ. 1998. Ritonavir. Clin Pharmacokinet. 35: 275–291. ArticlePubMed
- Ikegame S, Takeda M, Ohno S, Nakatsu Y, Nakanishi Y, et al. 2010. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. J Virol. 84: 372–379. ArticlePubMedPDF
- Iketani S, Ho DD. 2024. SARS-CoV-2 resistance to monoclonal antibodies and small-molecule drugs. Cell Chem Biol. 31: 632–657. ArticlePubMedPMC
- Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, et al. 2020a. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 580: 391–395. ArticlePDF
- Jiao J, Zhao G, Wang Y, Ren P, Wu M. 2020b. MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the inflammatory response and improves neurological outcomes in mice model of spinal cord injury. Front Mol Biosci. 7: 37.Article
- Kaminski JJ, Schattgen SA, Tzeng TC, Bode C, Klinman DM, et al. 2013. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol. 191: 3876–3883. ArticlePubMedPDF
- Kanneganti TD. 2020. Intracellular innate immune receptors: Life inside the cell. Immunol Rev. 297: 5–12. ArticlePubMedPMCPDF
- Karki R, Kanneganti TD. 2021. The 'cytokine storm': Molecular mechanisms and therapeutic prospects. Trends Immunol. 42: 681–705. ArticlePubMedPMC
- Karki R, Lee S, Mall R, Pandian N, Wang Y, et al. 2022. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci Immunol. 7: eabo6294. ArticlePubMed
- Karki R, Lee E, Place D, Samir P, Mavuluri J, et al. 2018. IRF8 regulates transcription of Naips for NLRC4 inflammasome activation. Cell. 173: 920–933. ArticlePubMedPMC
- Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, et al. 2021a. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 184: 149–168. Article
- Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RKS, et al. 2021b. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 37: 109858.Article
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, et al. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 23: 19–28. ArticlePubMed
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 441: 101–105. ArticlePubMedPDF
- Kawai T, Akira S. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 21: 317–337. ArticlePubMedPMC
- Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. 2008. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 584: 40–48. ArticlePubMed
- Kell AM, Gale M Jr. 2015. RIG-I in RNA virus recognition. Virology. 479–480: 110–121. ArticlePubMed
- Kesavardhana S, Malireddi RKS, Burton AR, Porter SN, Vogel P, et al. 2020. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. 295: 8325–8330. ArticlePubMedPMC
- Kim H, Seo JS, Lee SY, Ha KT, Choi BT, et al. 2020. AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun. 87: 765–776. ArticlePubMed
- Klück V, Jansen TLTA, Janssen M, Comarniceanu A, Efdé M, et al. 2020. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: An open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2: e270–e280. ArticlePubMedPMC
- Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 477: 592–595. ArticlePubMedPMCPDF
- Koh HS, Lee S, Lee HJ, Min JW, Iwatsubo T, et al. 2021. Targeting microRNA-485-3p blocks Alzheimer's disease progression. Int J Mol Sci. 22: 13136.ArticlePubMedPMC
- Kumari P, Russo AJ, Shivcharan S, Rathinam VA. 2020. AIM2 in health and disease: Inflammasome and beyond. Immunol Rev. 297: 83–95. ArticlePubMedPMCPDF
- Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, et al. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 1: aaf1507.Article
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 1: 398–401. ArticlePubMedPDF
- Kwak H, Lee E, Karki R. 2025. DNA sensors in metabolic and cardiovascular diseases: Molecular mechanisms and therapeutic prospects. Immunol Rev. 329: e13382.ArticlePubMed
- Lee S, Channappanavar R, Kanneganti TD. 2020. Coronaviruses: Innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 41: 1083–1099. ArticlePubMedPMC
- Lee S, Cho HJ, Ryu JH. 2021a. Innate immunity and cell death in Alzheimer's disease. ASN Neuro. 13: 17590914211051908.ArticlePubMedPMCPDF
- Lee S, Hirohama M, Noguchi M, Nagata K, Kawaguchi A. 2018. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J Virol. 92: e00344–18. ArticlePDF
- Lee S, Ishitsuka A, Kuroki T, Lin YH, Shibuya A, et al. 2021b. Arf6 exacerbates allergic asthma through cell-to-cell transmission of ASC inflammasomes. JCI Insight. 6: e141711. Article
- Lee S, Ishitsuka A, Noguchi M, Hirohama M, Fujiyasu Y, et al. 2019. Influenza restriction factor MxA functions as inflammasome sensor in the respiratory epithelium. Sci Immunol. 4: eaau4643. ArticlePubMed
- Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, et al. 2021c. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 597: 415–419. ArticlePubMedPMCPDF
- Lentini G, Fama A, De Gaetano GV, Coppolino F, Mahjoub AK, et al. 2023. Caspase-8 inhibition improves the outcome of bacterial infections in mice by promoting neutrophil activation. Cell Rep Med. 4: 101098.ArticlePubMedPMC
- Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, et al. 2012. Anthrax lethal factor cleavage of NLRP1 is required for activation of the inflammasome. PLoS Pathog. 8: e1002638. ArticlePubMedPMC
- Li W, Chen H, Sutton T, Obadan A, Perez DR. 2014. Interactions between the influenza A virus RNA polymerase components and retinoic acid-inducible gene I. J Virol. 88: 10432–10447. ArticlePubMedPMCPDF
- Li H, Guan Y, Liang B, Ding P, Hou X, et al. 2022a. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur J Pharmacol. 928: 175091.ArticlePubMed
- Li D, Wu M. 2021. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 6: 291.ArticlePubMedPMCPDF
- Li R, Zan Y, Sui K, Zhu S. 2022b. The latest breakthrough on NLRP6 inflammasome. Precis Clin Med. 5: pbac022.ArticlePubMedPMCPDF
- Liao KC, Mogridge J. 2013. Activation of the NLRP1B inflammasome by reduction of cytosolic ATP. Infect Immun. 81: 570–579. ArticlePubMedPMCPDF
- Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. 2008. Critical function for NAIP5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 9: 1171–1178. ArticlePubMedPMCPDF
- Liu G, Lu Y, Thulasi Raman SN, Xu F, Wu Q, et al. 2018. Nuclear-resident RIG-I senses viral replication inducing antiviral immunity. Nat Commun. 9: 3199.ArticlePubMedPMCPDF
- Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine. 42: 145–151. ArticlePubMed
- Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, et al. 2017. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 36: 2529–2543. ArticlePubMedPMC
- Malireddi RKS, Karki R, Sundaram B, Kancharana B, Lee S, et al. 2021. Inflammatory cell death, PANoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons. 5: 568–580. ArticlePubMedPDF
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, et al. 2015. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 16: 467–475. ArticlePubMedPMCPDF
- Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, et al. 2018a. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci USA. 115: E1530–E1539. Article
- Marchetti C, Swartzwelter B, Koenders MI, Azam T, Tengesdal IW, et al. 2018b. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther. 20: 169.ArticlePDF
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, et al. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440: 228–232. ArticlePubMedPDF
- Marshall JS, Warrington R, Watson W, Kim HL. 2018. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 14: 49.ArticlePubMedPMCPDF
- Martinon F, Burns K, Tschopp J. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol Cell. 10: 417–426. ArticlePubMed
- McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, et al. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4: e1000108.ArticlePubMedPMC
- McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. 2000. High-affinity interaction between Gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun. 68: 5525–5529. ArticlePubMedPMCPDF
- McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, et al. 2018. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci USA. 115: E6065–E6074. ArticlePubMedPMC
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 170: 5165–5175. ArticlePubMedPDF
- Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, et al. 2015. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci USA. 112: 5455–5460. ArticlePubMedPMC
- Oh J, Kim H, Lee J, Kim S, Shin S, et al. 2025. Korean Red ginseng enhances ZBP1-mediated cell death to suppress viral protein expression in host defense against influenza A virus. J Microbiol. 63: e2409007.ArticlePDF
- Oh S, Lee S. 2023. Recent advances in ZBP1-derived PANoptosis against viral infections. Front Immunol. 14: 1148727.ArticlePubMedPMC
- Oh S, Lee J, Oh J, Yu G, Ryu H, et al. 2023. Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. Cell Mol Immunol. 20: 1513–1526. ArticlePubMedPMCPDF
- Oliveira-Nascimento L, Massari P, Wetzler LM. 2012. The role of TLR2 in infection and immunity. Front Immunol. 3: 79.ArticlePubMedPMC
- Place DE, Lee S, Kanneganti TD. 2021. PANoptosis in microbial infection. Curr Opin Microbiol. 59: 42–49. ArticlePubMed
- Rasenack J, Zeuzem S, Feinman SV, Heathcote EJ, Manns M, et al. 2003. Peginterferon α-2a (40kD) [Pegasys®] improves HR-QOL outcomes compared with unmodified interferon α-2a [Roferon®-A]. Pharmacoeconomics. 21: 341–349. ArticlePubMed
- Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc Natl Acad Sci USA. 99: 2281–2286. ArticlePubMedPMC
- Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 11: 395–402. ArticlePubMedPMCPDF
- Rehwinkel J, Gack MU. 2020. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat Rev Immunol. 20: 537–551. ArticlePubMedPMCPDF
- Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, et al. 2013. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 305: 723–732. ArticlePubMedPDF
- Roth S, Cao J, Singh V, Tiedt S, Hundeshagen G, et al. 2021. Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity. 54: 648–659. ArticlePubMed
- Sánchez-Fernández A, Skouras DB, Dinarello CA, López-Vales R. 2019. OLT1177 (dapansutrile), a selective NLRP3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front Immunol. 10: 2578.ArticlePubMedPMC
- Sharma BR, Karki R, Kanneganti TD. 2019. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 49: 1998–2011. ArticlePubMedPMCPDF
- Smith DA, Conkling P, Richards DA, Nemunaitis JJ, Boyd TE, et al. 2014. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother. 63: 787–796. ArticlePubMedPMCPDF
- Song J, Li M, Li C, Liu K, Zhu Y, et al. 2022. Friend or foe: RIG-I like receptors and diseases. Autoimmun Rev. 21: 103161.ArticlePubMedPMC
- Sridharan H, Ragan KB, Guo H, Gilley RP, Landsteiner VJ, et al. 2017. Murine cytomegalovirus IE3-dependent transcription is required for DAI/ZBP1-mediated necroptosis. EMBO Rep. 18: 1429–1441. ArticlePubMedPMC
- Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, et al. 2019. Aggregated tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded tau pathology in vivo. Acta Neuropathol. 137: 599–617. ArticlePubMedPMCPDF
- Świerczyńska M, Mirowska-Guzel DM, Pindelska E. 2022. Antiviral drugs in influenza. Int J Environ Res Public Health. 19: 3018.ArticlePubMedPMC
- Takeda K, Akira S. 2015. Toll-like receptors. Curr Protoc Immunol. 109: 14.ArticlePDF
- Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, et al. 2016. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 20: 674–681. ArticlePubMedPMC
- Uysal E, Dokur M, Kucukdurmaz F, Altinay S, Polat S, et al. 2022. Targeting the panoptosome with 3,4-methylenedioxy-β-nitrostyrene reduces PANoptosis and protects the kidney against renal ischemia-reperfusion injury. J Invest Surg. 35: 1824–1835. ArticlePubMed
- van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, et al. 2022. A guide to immunotherapy for COVID-19. Nat Med. 28: 39–50. ArticlePubMedPDF
- Vance RE. 2015. The NAIP/NLRC4 inflammasomes. Curr Opin Immunol. 32: 84–89. ArticlePubMed
- Venuprasad K, Theiss AL. 2021. NLRP6 in host defense and intestinal inflammation. Cell Rep. 35: 109043.ArticlePubMedPMC
- Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, et al. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 79: 14355–14370. ArticlePubMedPMCPDF
- Wang JP, Cerny A, Asher DR, Kurt-Jones EA, Bronson RT, et al. 2010. MDA5 and MAVS mediate type I interferon responses to Coxsackie B virus. J Virol. 84: 254–260. ArticlePubMedPDF
- Wang XQ, Wan HQ, Wei XJ, Zhang Y, Qu P. 2016. CLI-095 decreases atherosclerosis by modulating foam cell formation in apolipoprotein E-deficient mice. Mol Med Rep. 14: 49–56. ArticlePubMedPMC
- Woollard SM, Kanmogne GD. 2015. Maraviroc: A review of its use in HIV infection and beyond. Drug Des Devel Ther. 9: 5447–5468. ArticlePubMedPMC
- Yin X, Riva L, Pu Y, Martin-Sancho L, Kanamune J, et al. 2021. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 34: 108628.ArticlePubMedPMC
- Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, et al. 2012. Structural basis of TLR5-flagellin recognition and signaling. Science. 335: 859–864. ArticlePubMedPMC
- Yu G, Choi YK, Lee S. 2024. Inflammasome diversity: Exploring novel frontiers in the innate immune response. Trends Immunol. 45: 248–258. ArticlePubMed
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, et al. 2016. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 352: 1232–1236. ArticlePubMedPMC
- Zeng W, Wu D, Sun Y, Suo Y, Yu Q, et al. 2021. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci Rep. 11: 19305.ArticlePubMedPMCPDF
- Zhao K, Du J, Peng Y, Li P, Wang S, et al. 2018. LINE1 contributes to autoimmunity through both RIG-I- and MDA5-mediated RNA sensing pathways. J Autoimmun. 90: 105–115. ArticlePubMed
- Zhong Y, Kinio A, Saleh M. 2013. Functions of NOD-like receptors in human diseases. Front Immunol. 4: 333.ArticlePubMedPMC
- Zhu S, Ding S, Wang P, Wei Z, Pan W, et al. 2017. NLRP9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 546: 667–670. ArticlePubMedPMC
References
Figure & Data
References
Citations

- A new fucosylated glucuronoxylomannan from the fruit bodies of Tremella aurantia: structural characterization and immunoenhancing activity on seasonal influenza mRNA vaccine
Jing Chen, Yuan Ma, Zhi-Min Rao, Song-Lin Jiang, Ying-Jun Lou, Karim Malik, Arman Chowdhury, Hua-Zhong Ying, Chen-Huan Yu
Carbohydrate Polymers.2026; 373: 124660. CrossRef - Z-DNA interaction proteins - insights from ChIP-seq data
Michaela Dobrovolná, Václav Brázda
Biochemical and Biophysical Research Communications.2025; 790: 152910. CrossRef - AIM2 drives inflammatory cell death and monkeypox pathogenesis
Jueun Oh, Yun-Ho Hwang, Jihye Lee, Cheong Seok, SuHyeon Oh, Hye Yoon Kim, Nabukenya Mariam, Jaeyoung Ahn, GyeongJu Yu, Jaewoo Park, Hayeon Kim, Suhyun Kim, Seyun Shin, Min-Chul Jung, Jinwoo Gil, Joo Sang Lee, Young Ki Choi, Dokeun Kim, Daesik Kim, You-Jin
Cellular & Molecular Immunology.2025; 22(12): 1615. CrossRef
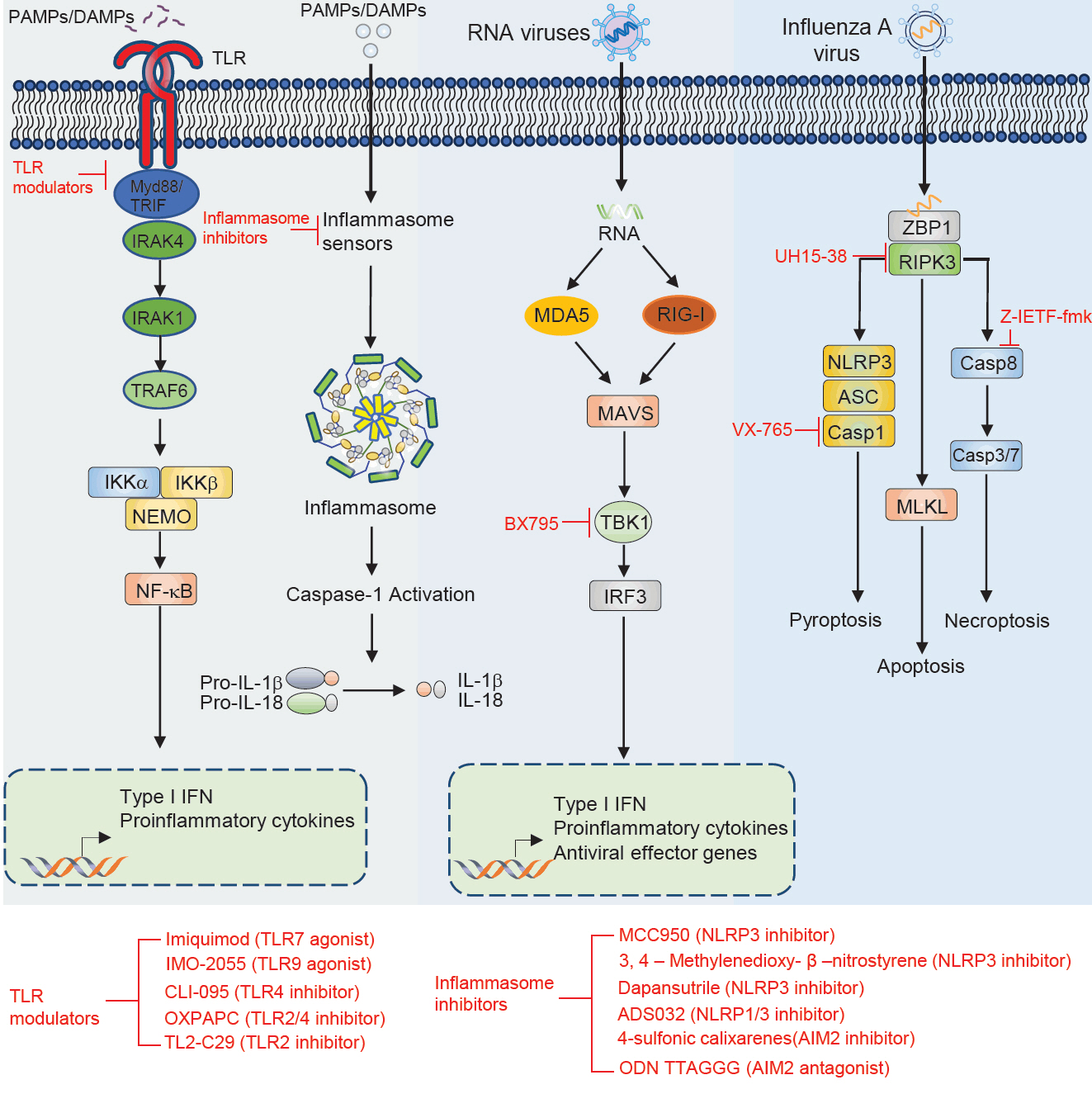
Fig. 1.
| Therapeutic molecule | Target | Disease | Effect | Outcome | Reference |
|---|---|---|---|---|---|
| Imiquimod | TLR7 | Tumor | Agonist | Induce the production of cytokine | |
| IMO-2055 | TLR9 | Tumor | Agonist | Enhance antitumor efficacy | |
| CLI-095 | TLR4 | Atherosclerosis | Inhibitor | Suppress LPS induced inflammation | |
| OXPAPC | TLR2, TLR4 | Sepsis shock | Inhibitor | Inhibits non-canonical pyroptosis | |
| TL2-C29 | TLR2 | Hepatitis C virus | Inhibitor | Inhibitor of TLR2/1 signaling | |
| MCC950 | NLRP3 | Inflammatory diseases (atherosclerosis, myocardial fibrosis, spinal cord injury, neurological disorders, intestinal inflammation) | Inhibitor | Alleviates symptoms of associated inflammatory conditions | |
| 3,4-Methylenedioxy-β-nitrostyrene | NLRP3 | Renal ischemia | Inhibitor | Protects from renal ischemia | |
| Dapansutrile | NLRP3 | Autoimmune encephalomyelitis, acute arthritis | Inhibitor | Atternuates clinical signs and improves prognosis | |
| ADS032 | NLRP1, NLRP3 | IAV-induced pulmonary inflammation and disease severity | Inhibitor | reduces acute silicosis-associated pulmonary inflammation | |
| 4-Sulfonic calixarenes | AIM2 | Post-stroke immunosuppression | Inhibitor | AIM2-dependent post-stroke T cell death inhibition | |
| ODN TTAGGG | AIM2 | MCMV and L. monocytogenes | Antagonist | Blocks AIM2 inflammasome activation in response to cytosolic dsDNA | |
| UH15-38 | RIPK3 | Blocked IAV-triggered necroptosis in alveolar epithelial cells in vivo | Inhibitor | UH15-38 ameliorated lung inflammation and prevented mortality | |
| z-IETF-fmk | Caspase8 | Lethal bacterial peritonitis and pneumonia | Inhibitor | z-IETD-fmk induces pro-inflammatory cytokine productin in neutrophils but not in macrophages | |
| VX-765 | Caspase1 | CNS disease | Inhibitor | Reduces CNS inflammation, prevents axonal injury, improves neurobehavioral in EAE |
Table 1.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article


