Articles
- Page Path
- HOME > J. Microbiol > Volume 63(6); 2025 > Article
-
Full article
Inhibiting kinesin family member 20A disrupts Zika virus entry by blocking internalization - Jeonghyeon Lee†, Younghyun Lim†, Hyeong-Rae Kim, Yong-Bin Cho, In-Gu Lee, Young-Jin Seo*
-
Journal of Microbiology 2025;63(6):e2503008.
DOI: https://doi.org/10.71150/jm.2503008
Published online: June 30, 2025
Department of Life Science, Chung-Ang University, Seoul 06974, Republic of Korea
- *Correspondence Young-Jin Seo yjseo@cau.ac.kr
- †These authors contributed equally to this work.
• Received: March 13, 2025 • Revised: May 15, 2025 • Accepted: May 20, 2025
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,306 Views
- 54 Download
ABSTRACT
- Zika virus, a mosquito-borne virus, is associated with congenital birth defects and neurological complications. However, despite its significant public health threat, no approved vaccines or antiviral treatments are currently available. Therefore, this study aims to identify kinesin family member 20A as a key host factor promoting Zika virus life cycle. The elevated expression of kinesin family member 20A following Zika virus infection suggests its role in the viral life cycle. Suppressing its expression through gene silencing or inhibiting its function with a small-molecule inhibitor significantly reduced viral infectivity in host cells. Furthermore, kinesin family member 20A is essential for facilitating viral internalization, a key step in the entry step. These findings suggest its significance in the Zika virus life cycle and highlight its potential as a novel therapeutic target for the Zika virus.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government [grant number RS-2025-00556948].
Conflict of Interest
The authors have no conflict of interest to report.
Supplementary Information
Fig. S1.
Fig. S2.
Fig. 1.Zika virus infection increases the expression of KIF20A. (A–C) Vero E6 cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 0.1 or 1. Cells were harvested at 6 h post-infection (hpi), and mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified using qRT-PCR. (A) Relative KIF20A mRNA levels normalized to GAPDH. (B, C) Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. (D–F) Vero E6 cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 1. Cells were harvested at 0, 2, 3, 4, 5, and 6 h post-infection, and mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified using qRT-PCR. (D) Relative KIF20A mRNA levels normalized to GAPDH. (E, F) Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Data are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
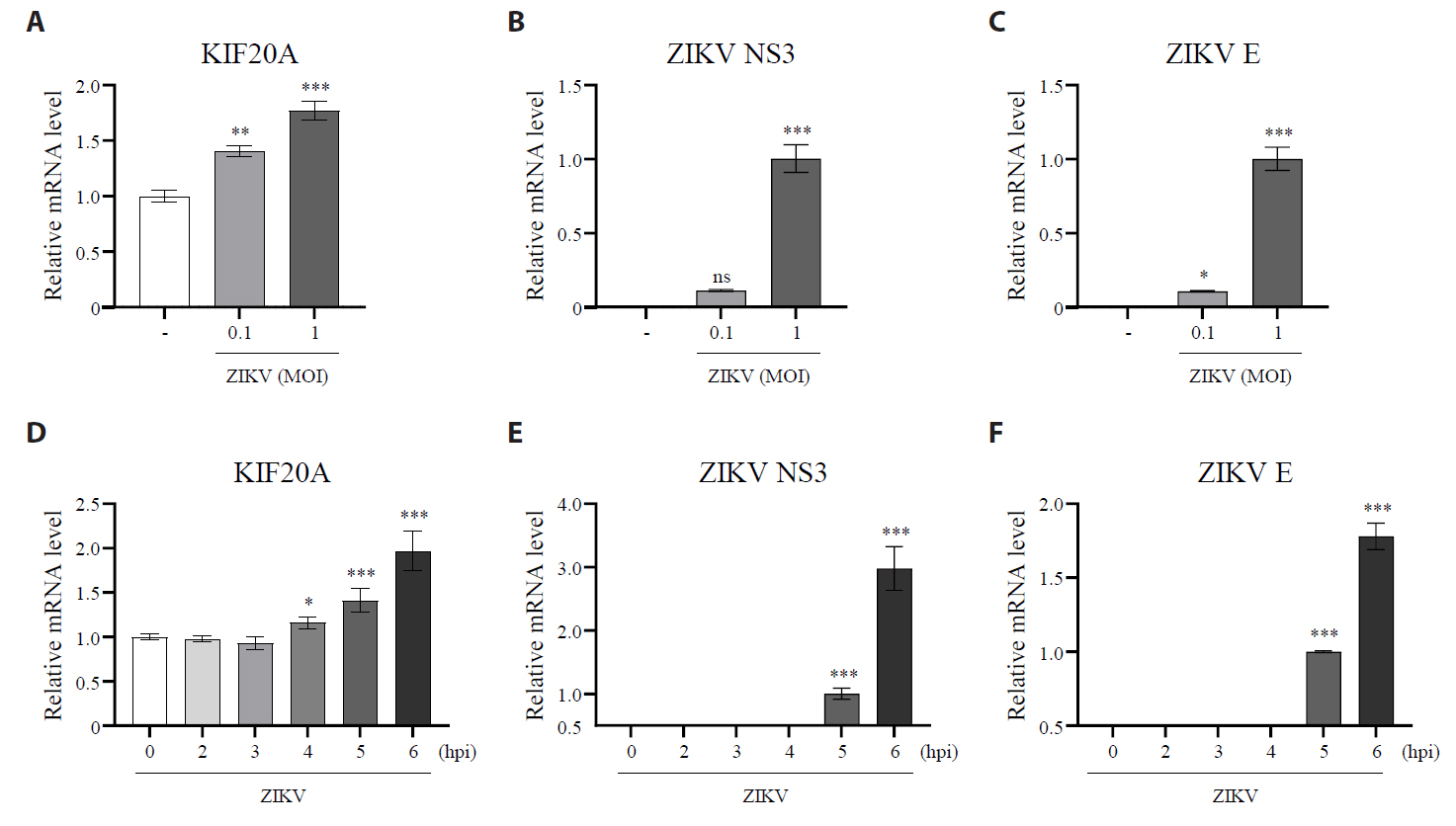

Fig. 2.KIF20A knockdown attenuates Zika virus infection. (A–C) Vero E6 cells were transfected with either control scrambled small interfering RNA (siRNA) or one of three different siRNA targeting KIF20A (siRNA #1, #2, and #3). After transfection, cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 5. At 6 h post-infection (hpi), total RNA was extracted, while the mRNA levels of KIF20A and ZIKV NS3 were quantified using qRT-PCR. (A) Relative KIF20A expressions normalized to GAPDH. (B) Relative mRNA levels of ZIKV E normalized to GAPDH. (C) KIF20A protein levels analyzed using western blot. (D–F) HEK293T cells were transduced with lentivirus encoding either control shRNA or KIF20A-targeting shRNA. Transduction efficiency was confirmed using a GFP reporter and selected with puromycin. Stable cell lines were infected with ZIKV at an MOI of 0.5. At 6 hpi, total RNA was extracted, while the mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified. (D) Relative mRNA levels of KIF20A normalized to GAPDH. (E, F) Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences using an as determined using an analysis of variance (ANOVA) (*P < 0.1, **P < 0.01, ***P < 0.001), or unpaired t-test (***P < 0.001).
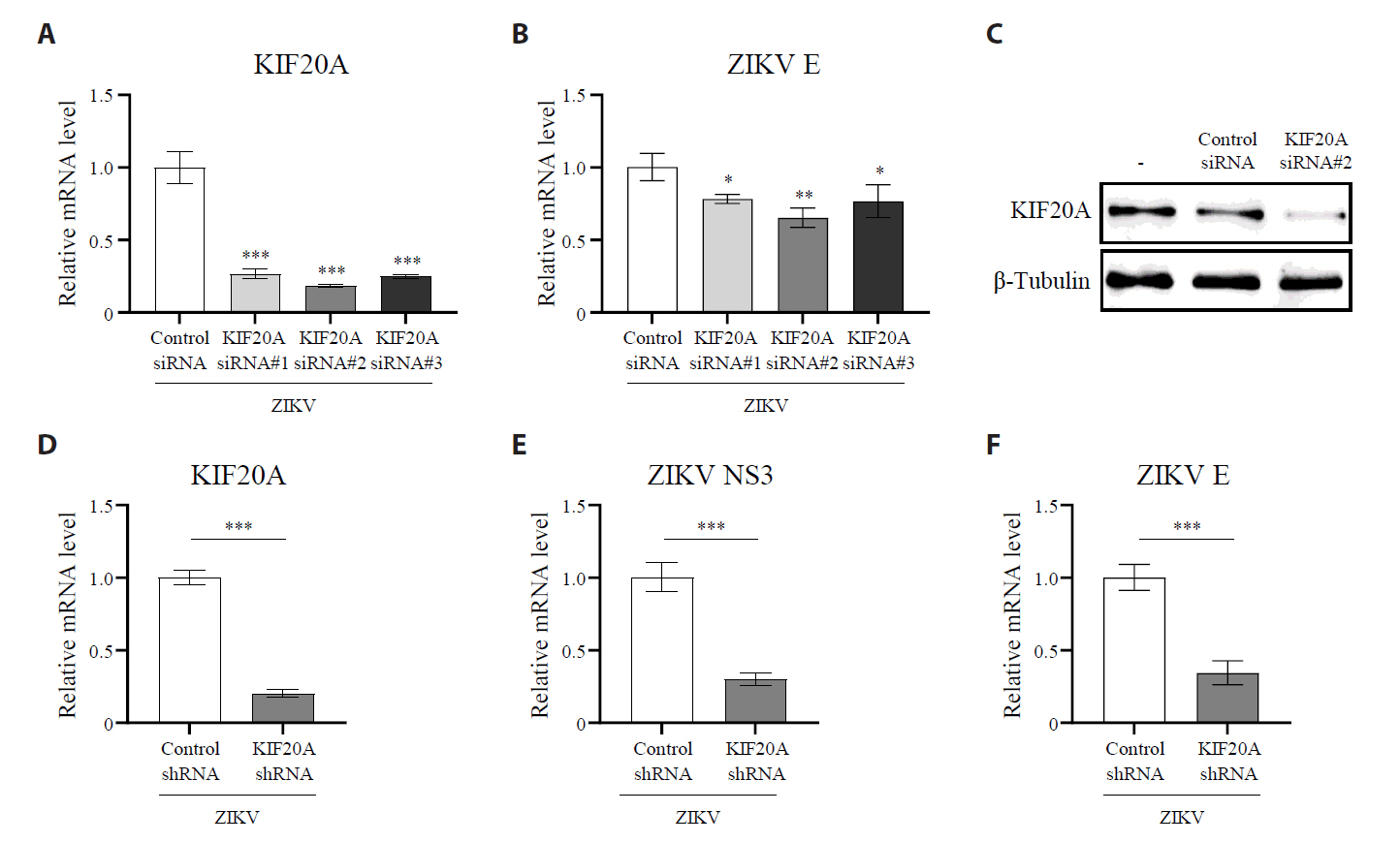

Fig. 3.KIF20A inhibition suppresses Zika virus infection. (A) Vero E6 cells were treated with paprotrain at concentrations ranging from 100 to 1 µg/ml. After 12 h, cell viability was determined using the trypan blue exclusion assay. (B–E) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.5 and co-treated with either 20 µg/ml paprotrain or 50 µM chloroquine (CQ) for 18 h. (B, C) Relative mRNA levels of ZIKV NS3 (B) and E (C) normalized to GAPDH. (D) ZIKV E protein levels analyzed using Western blot. (E) Viral titers in the culture medium quantified using a focus-forming assay. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
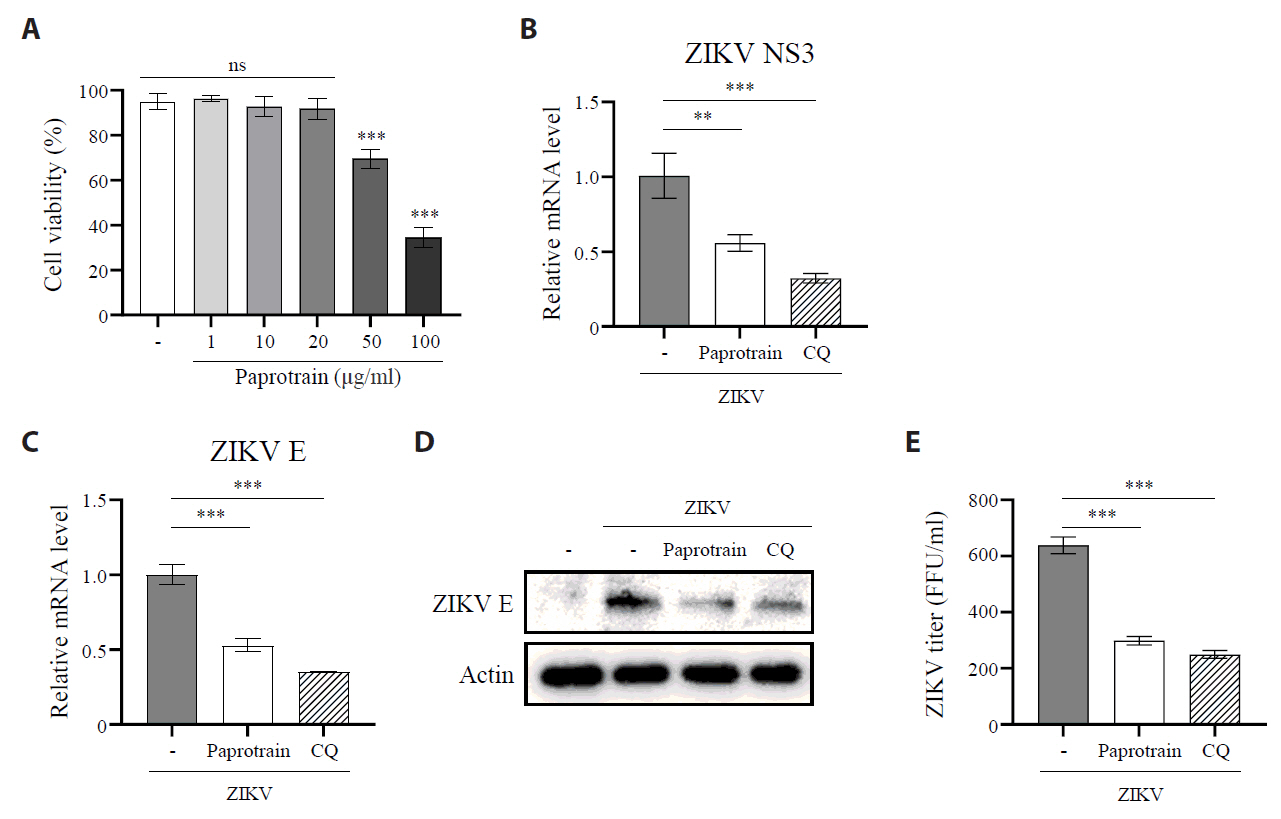

Fig. 4.KIF20A inhibition reduces the number of ZIKV-infected cells. (A, B) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 5, with or without 20 µg/ml paprotrain. After 18 hpi, infected cells were fixed, permeabilized, and stained with an anti-ZIKV E protein antibody for flow cytometric analysis. (A) Representative histograms illustrating the proportion of ZIKV E–positive (infected) cells. (B) Percentages of infected cells. (C, D) Vero E6 cells infected with ZIKV at an MOI of 5, with or without 20 µg/ml paprotrain. After 18 hpi, infected cells were fixed, permeabilized, and stained with DAPI and an anti-ZIKV E protein antibody for immunofluorescence microscopy. (C) Representative images showing infected cells. (D) Quantification from three independent experiments, where infected cells were defined as the number of ZIKV E–positive nuclei (co-localized with DAPI) divided by the total number of DAPI–stained nuclei. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (*P < 0.01, ***P < 0.001).

Fig. 5.KIF20A inhibition attenuates Zika virus infection on host cells at an early time point. (A) Schematic representation of treatment conditions: virus treatment (VT), cell treatment (CT), co-treatment (Co-T), and post-treatment (PT). (B) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 0.5. At 18 h post-infection (hpi), cells were harvested and lysed, and ZIKV E protein levels were analyzed using Western blot. (C) Schematic diagram of the time-of addition assay. Cells were treated with 20 µg/ml paprotrain at various time points relative to ZIKV infection. For pretreatment, cells received paprotrain 6 h and 3 h before infection. For co-treatment and post-treatment, paprotrain was added at 0 h (co-treatment) or 4 h, 7 h, and 16 h post-infection. (D) Vero E6 cells were infected with ZIKV at an MOI of 0.5. At 18 hpi, cells were harvested and lysed, while ZIKV E protein levels were analyzed using Western blot. All experiments were performed at least twice. (E) Vero E6 cells were infected with ZIKV at an MOI of 1, and relative vRNA levels of ZIKV NS3 were normalized to GAPDH at each time point after ZIKV infection. (F) Vero E6 cells were infected with ZIKV at an MOI of 1, and treated with 20 µg/ml paprotrain at 7 hpi for 1 h. Relative vRNA levels of ZIKV NS3 were normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (***P < 0.001).
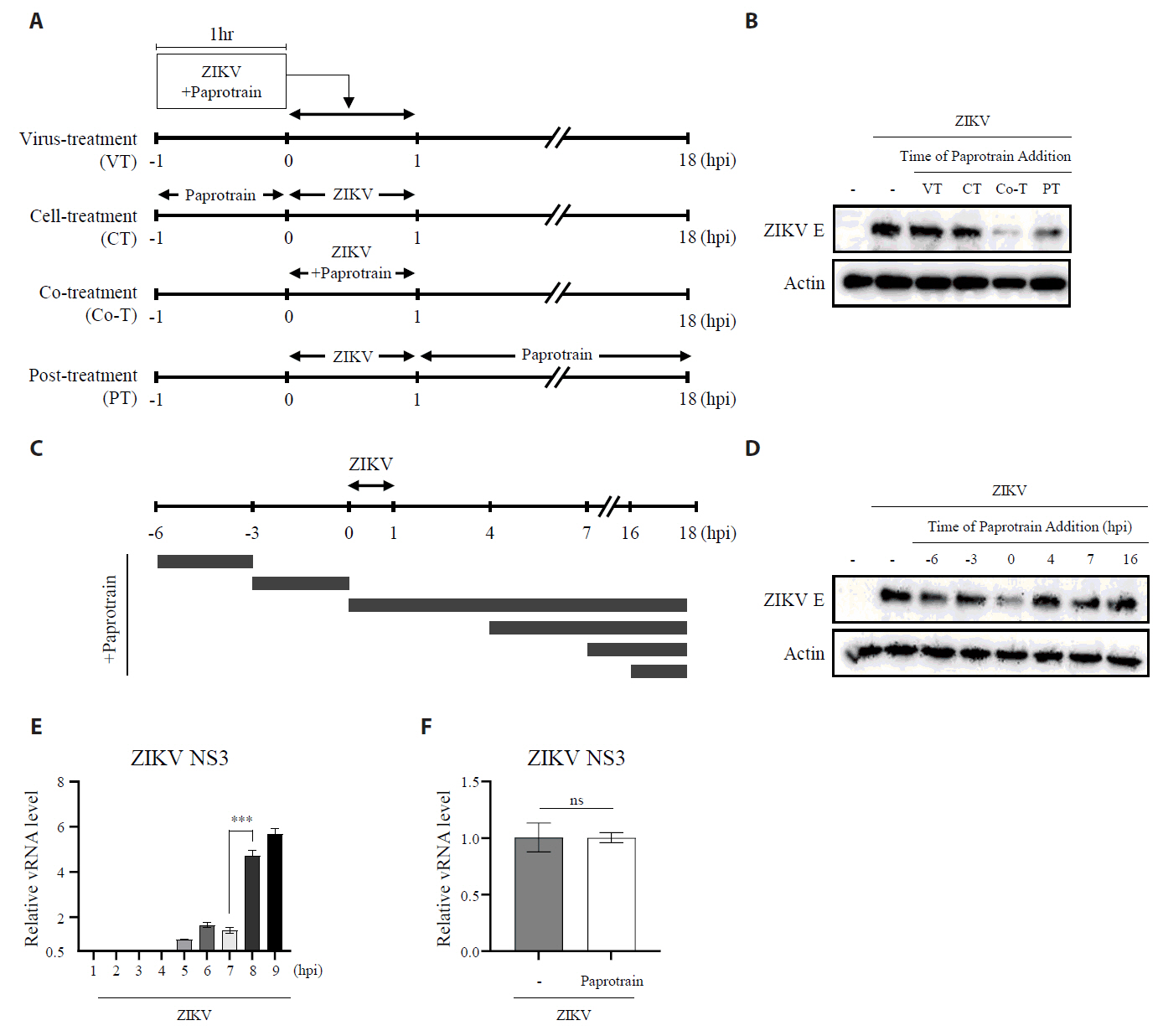

Fig. 6.The inhibition of KIF20A interferes with the Zika virus internalization during entry stages. (A) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV attachment. (B, C) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 10 with or without 20 µg/ml paprotrain at 4°C. After 1 h, cells were washed five times with cold PBS. The samples were then immediately processed for total RNA extraction. Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. (D) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV entry. (E, F) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 10 for 1 h at 4°C. Following infection, cells were washed five times with cold PBS, followed by treatment with 20 µg/ml paprotrain. After incubation at 37°C for 1 h, the cells were washed with cold PBS and treated with proteinase K (1 mg/ml) for 45 min at 4°C to remove surface-bound but non-internalized virus. The samples were then immediately processed for total RNA extraction. Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
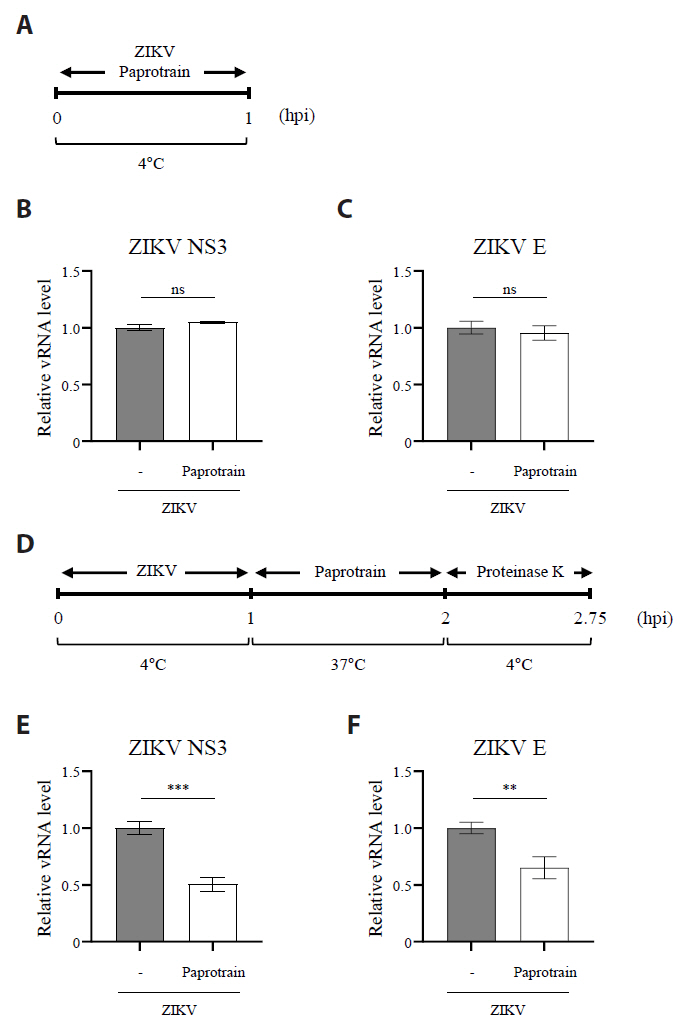

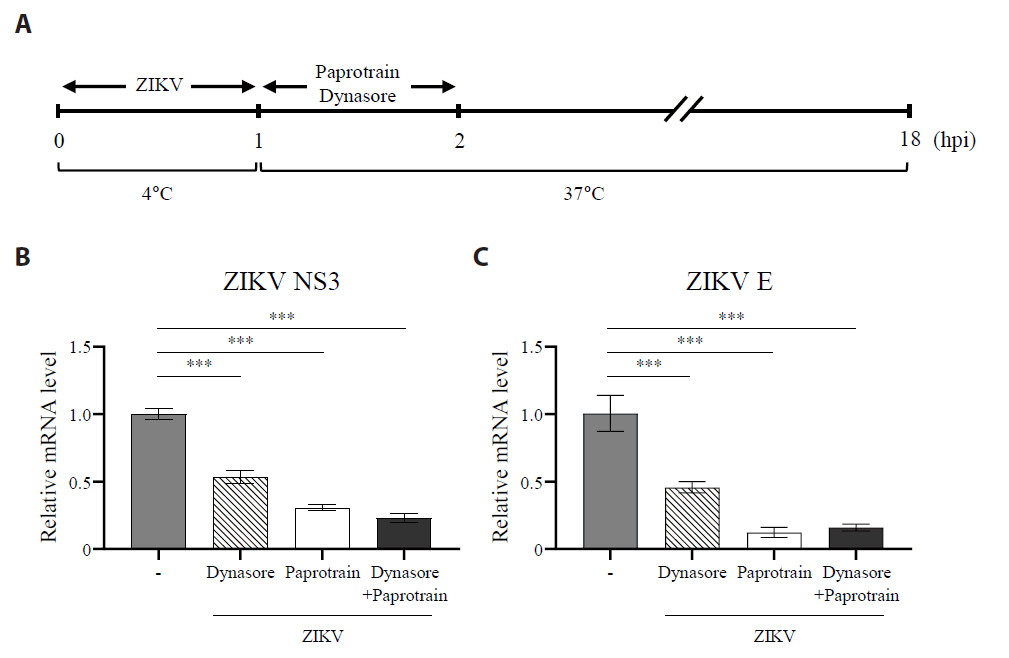
Fig. 7.The inhibition of KIF20A suppresses clathrin-mediated entry of ZIKV. (A) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV clathrin-mediated endocytosis (CME) pathway. (B, C) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.5 at 4°C. After 1 h, cells were washed three times with PBS and subsequently treated with paprotrain (20 µg/ml), dynasore (50 µM), or a combination of paprotrain (20 µg/ml) and dynasore (50 µM). At 18 h post infection (hpi), the levels of viral RNA were measured. Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).

- Bekerman E, Einav S. 2015. Combating emerging viral threats. Science. 348: 282–283. ArticlePubMedPMC
- Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, et al. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 375: 2321–2334. ArticlePubMedPMC
- Cho YB, Hong S, Kang KW, Kang JH, Lee SM, et al. 2020. Selective and ATP-competitive kinesin KIF18A inhibitor suppresses the replication of influenza A virus. J Cell Mol Med. 24: 5463–5475. ArticlePubMedPMCPDF
- Dharan A, Campbell EM. 2018. Role of microtubules and microtubule-associated proteins in HiV-1 infection. J Virol. 92: e00085–18. ArticlePMCPDF
- Diab A, Foca A, Fusil F, Lahlali T, Jalaguier P, et al. 2017. Polo-like-kinase 1 is a proviral host factor for hepatitis B virus replication. Hepatology. 66: 1750–1765. ArticlePubMedPDF
- Döhner K, Sodeik B. 2005. The role of the cytoskeleton during viral infection. Curr Top Microbiol Immunol. 285: 67–108. ArticlePubMed
- DuRaine G, Wisner TW, Howard P, Johnson DC. 2018. Kinesin-1 proteins KIF5A, -5B, and -5C promote anterograde transport of herpes simplex virus enveloped virions in axons. J Virol. 92: e01269–18. ArticlePubMedPMCPDF
- Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 374: 601–604. ArticlePubMed
- Gennerich A, Vale RD. 2009. Walking the walk: how kinesin and dynein coordinate their steps. Curr Opin Cell Biol. 21: 59–67. ArticlePubMedPMC
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. 2009. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 10: 682–696. ArticlePubMedPDF
- Jeon H, Lim Y, Lee IG, Kim DI, Kim KP, et al. 2022. Inhibition of KIF20A suppresses the replication of influenza A virus by inhibiting viral entry. J Microbiol. 60: 1113–1121. ArticlePubMedPDF
- Jouvenet N, Monaghan P, Way M, Wileman T. 2004. Transport of african swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J Virol. 78: 7990–8001. ArticlePubMedPMCPDF
- Kamiyama N, Soma R, Hidano S, Watanabe K, Umekita H, et al. 2017. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antiviral Res. 146: 1–11. ArticlePubMedPMC
- Kim DI, Kang JH, Kim EH, Seo YJ. 2021. KIF11 inhibition decreases cytopathogenesis and replication of influenza A virus. Mol Cell Toxicol. 17: 201–212. ArticlePDF
- Kim JA, Seong RK, Kumar M, Shin OS. 2018. Favipiravir and ribavirin inhibit replication of asian and african strains of Zika virus in different cell models. Viruses. 10: 72.ArticlePubMedPMC
- Lukic Z, Dharan A, Fricke T, Diaz-Griffero F, Campbell EM. 2014. HIV-1 uncoating is facilitated by dynein and kinesin 1. J Virol. 88: 13613–13625. ArticlePubMedPMCPDF
- Madejon A, Sheldon J, Francisco-Recuero I, Perales C, Dominguez-Beato M, et al. 2015. Hepatitis C virus-mediated Aurora B kinase inhibition modulates inflammatory pathway and viral infectivity. J Hepatol. 63: 312–319. ArticlePubMed
- Malikov V, da Silva ES, Jovasevic V, Bennett G, de Souza Aranha Vieira DA, et al. 2015. HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus. Nat Commun. 6: 6660.ArticlePubMedPDF
- Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, et al. 2017. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 18: 324–333. ArticlePubMed
- Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 6: 363–374. ArticlePubMedPMCPDF
- Miner JJ, Diamond MS. 2017. Zika virus pathogenesis and tissue tropism. Cell Host Microbe. 21: 134–142. ArticlePubMedPMC
- Miserey-Lenkei S, Bousquet H, Pylypenko O, Bardin S, Dimitrov A, et al. 2017. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat Commun. 8: 1254.ArticlePubMedPMCPDF
- Peck KM, Lauring AS. 2018. Complexities of viral mutation rates. J Virol. 92: e01031–17. ArticlePubMedPMCPDF
- Pérez-Olais JH, Ruiz-Jiménez F, Calderón-Garcia EJ, de Jesús-González LA, Hernández-Rivas R, et al. 2019. The activity of Aurora kinase B is required for dengue virus release. Virus Res. 274: 197777.ArticlePubMed
- Persaud M, Martinez-Lopez A, Buffone C, Porcelli SA, Diaz-Griffero F. 2018. Infection by Zika virus requires the transmembrane protein AXL, endocytosis and low pH. Virology. 518: 301–312. ArticlePubMed
- Ramos da Silva S, Cheng F, Huang IC, Jung JU, Gao SJ. 2019. Efficiencies and kinetics of infection in different cell types/lines by African and Asian strains of Zika virus. J Med Virol. 91: 179–189. ArticlePubMedPDF
- Ramos-Nascimento A, Kellen B, Ferreira F, Alenquer M, Vale-Costa S, et al. 2017. KIF13A mediates trafficking of influenza A virus ribonucleoproteins. J Cell Sci. 130: 4038–4050. ArticlePubMedPDF
- Sathish N, Zhu FX, Yuan Y. 2009. Kaposi's sarcoma-associated herpesvirus ORF45 interacts with kinesin-2 transporting viral capsid-tegument complexes along microtubules. PLoS Pathog. 5: e1000332. ArticlePubMedPMC
- She ZY, Li YL, Lin Y, Lu MH, Wei YL, et al. 2020. Kinesin-6 family motor KIF20A regulates central spindle assembly and acrosome biogenesis in mouse spermatogenesis. Biochim Biophys Acta Mol Cell Res. 1867: 118636.ArticlePubMed
- Shiryaev SA, Mesci P, Pinto A, Fernandes I, Sheets N, et al. 2017. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci Rep. 7: 15771.ArticlePubMedPMCPDF
- Smyk JM, Szydlowska N, Szulc W, Majewska A. 2022. Evolution of influenza viruses-drug resistance, treatment options, and prospects. Int J Mol Sci. 23: 12244.ArticlePubMedPMC
- Strunze S, Engelke MF, Wang IH, Puntener D, Boucke K, et al. 2011. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe. 10: 210–223. ArticlePubMed
- Tajik S, Farahani AV, Ardekani OS, Seyedi S, Tayebi Z, et al. 2024. Zika virus tropism and pathogenesis: understanding clinical impacts and transmission dynamics. Virol J. 21: 271.ArticlePubMedPMCPDF
- Tcherniuk S, Skoufias DA, Labriere C, Rath O, Gueritte F, et al. 2010. Relocation of Aurora B and survivin from centromeres to the central spindle impaired by a kinesin-specific MKLP-2 inhibitor. Angew Chem Int Ed Engl. 49: 8228–8231. ArticlePubMed
- Visher E, Whitefield SE, McCrone JT, Fitzsimmons W, Lauring AS. 2016. The mutational robustness of influenza A virus. PLoS Pathog. 12: e1005856. ArticlePubMedPMC
- Wang S, Zhang Q, Tiwari SK, Lichinchi G, Yau EH, et al. 2020. Integrin αvβ5 internalizes Zika virus during neural stem cells infection and provides a promising target for antiviral therapy. Cell Rep. 30: 969–983. ArticlePubMedPMC
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, et al. 2016. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 130: 69–80. ArticlePubMedPMC
- Wu WD, Yu KW, Zhong N, Xiao Y, She ZY. 2019. Roles and mechanisms of Kinesin-6 KIF20A in spindle organization during cell division. Eur J Cell Biol. 98: 74–80. ArticlePubMed
- Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, et al. 2018. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 4: 31.ArticlePubMedPMCPDF
- Zhang Y, Liu J, Peng X, Zhu CC, Han J, et al. 2014. KIF20A regulates porcine oocyte maturation and early embryo development. PLoS One. 9: e102898. ArticlePubMedPMC
- Zhou D, Hayashi T, Jean M, Kong W, Fiches G, et al. 2020. Inhibition of Polo-like kinase 1 (PLK1) facilitates the elimination of HIV-1 viral reservoirs in CD4+ T cells ex vivo. Sci Adv. 6: eaba1941. ArticlePubMedPMC
- Zhu Z, Jin Z, Zhang H, Zhang M, Sun D. 2020. Knockdown of Kif20a inhibits growth of tumors in soft tissue sarcoma in vitro and in vivo. J Cancer. 11: 5088–5098. ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

Inhibiting kinesin family member 20A disrupts Zika virus entry by blocking internalization
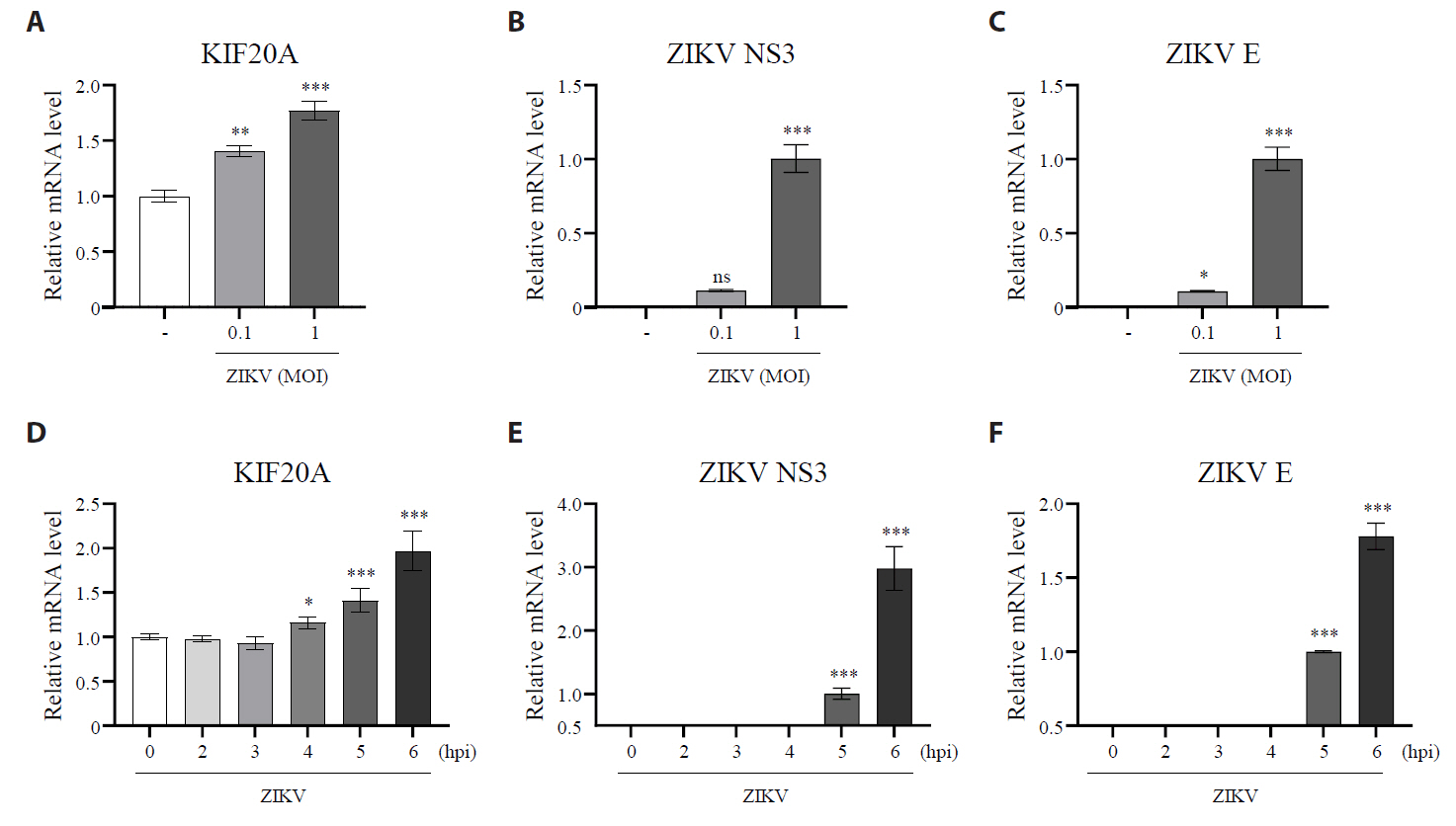
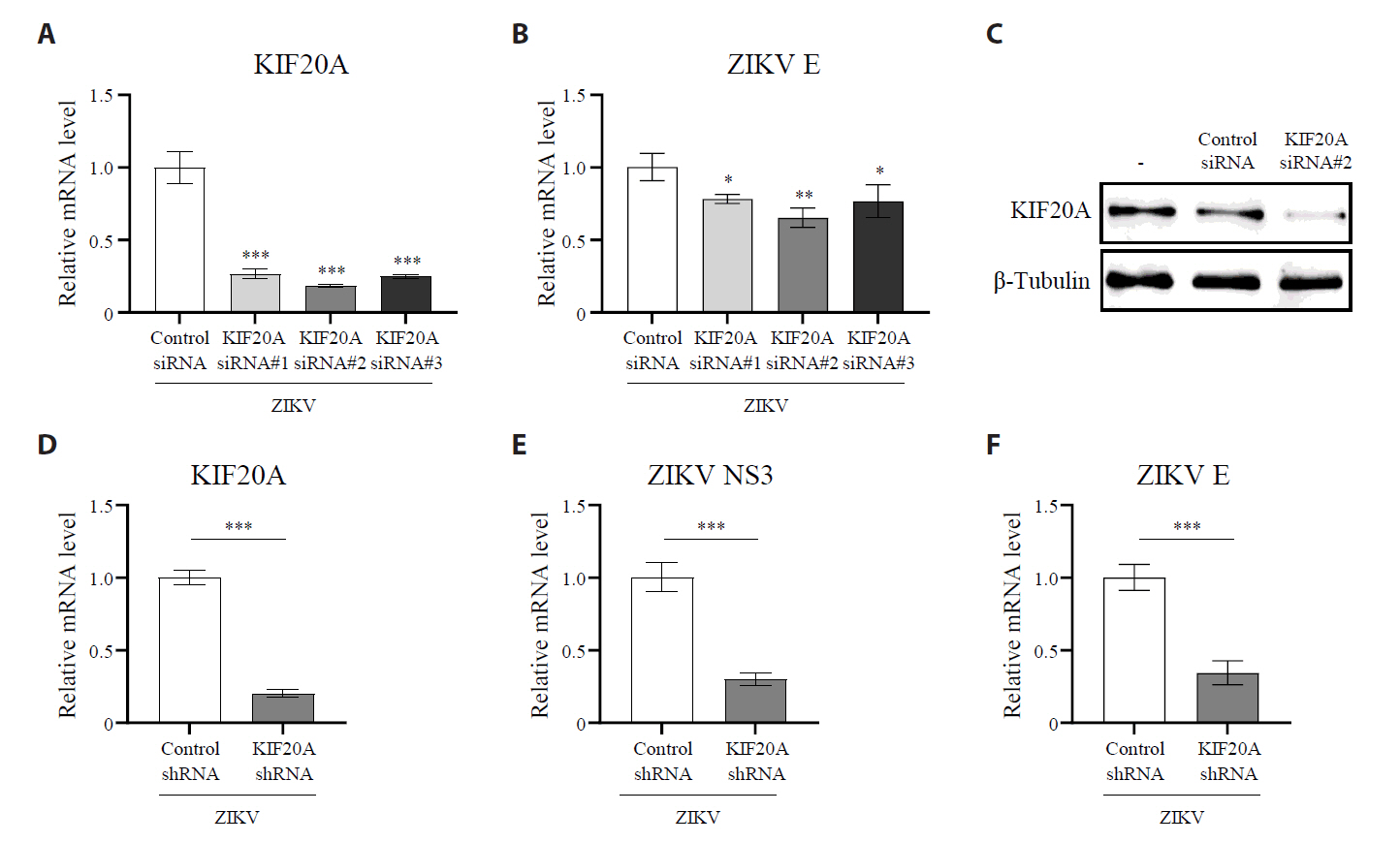
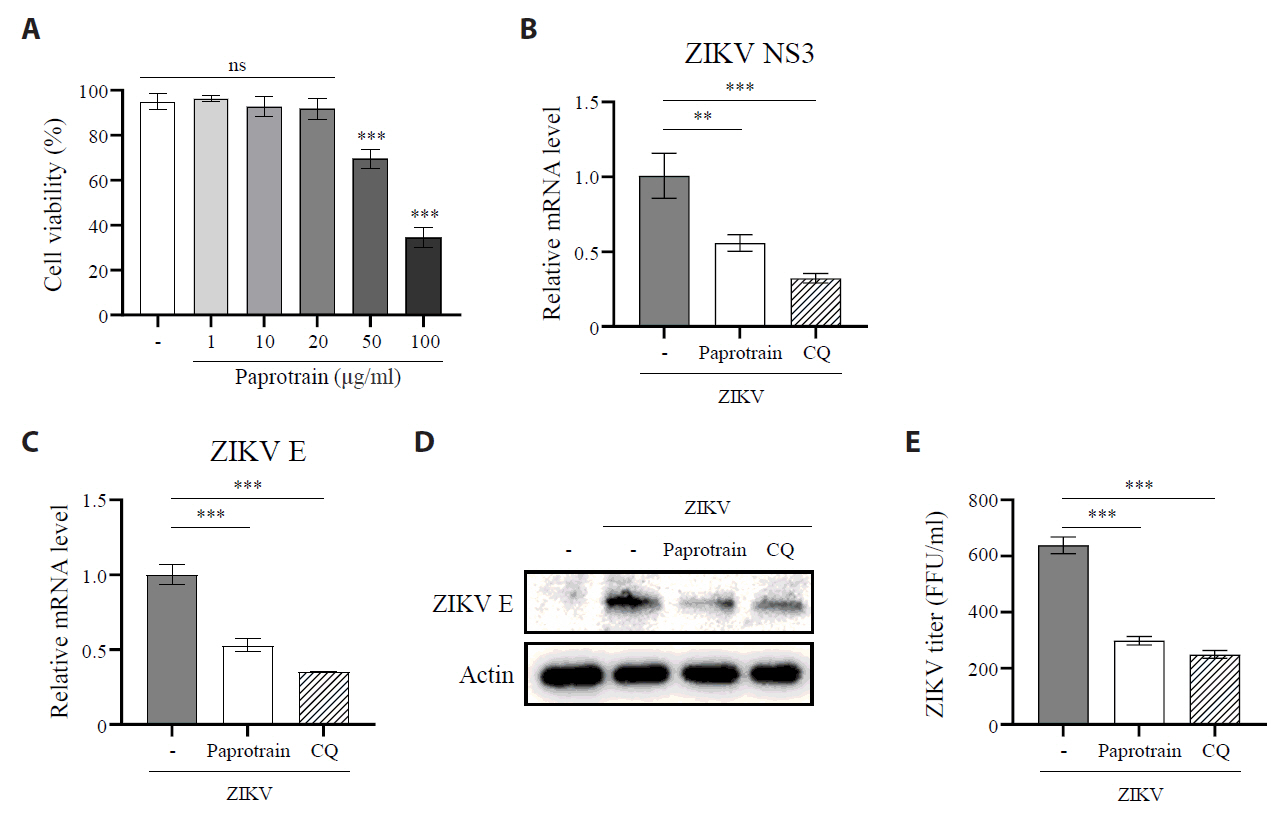
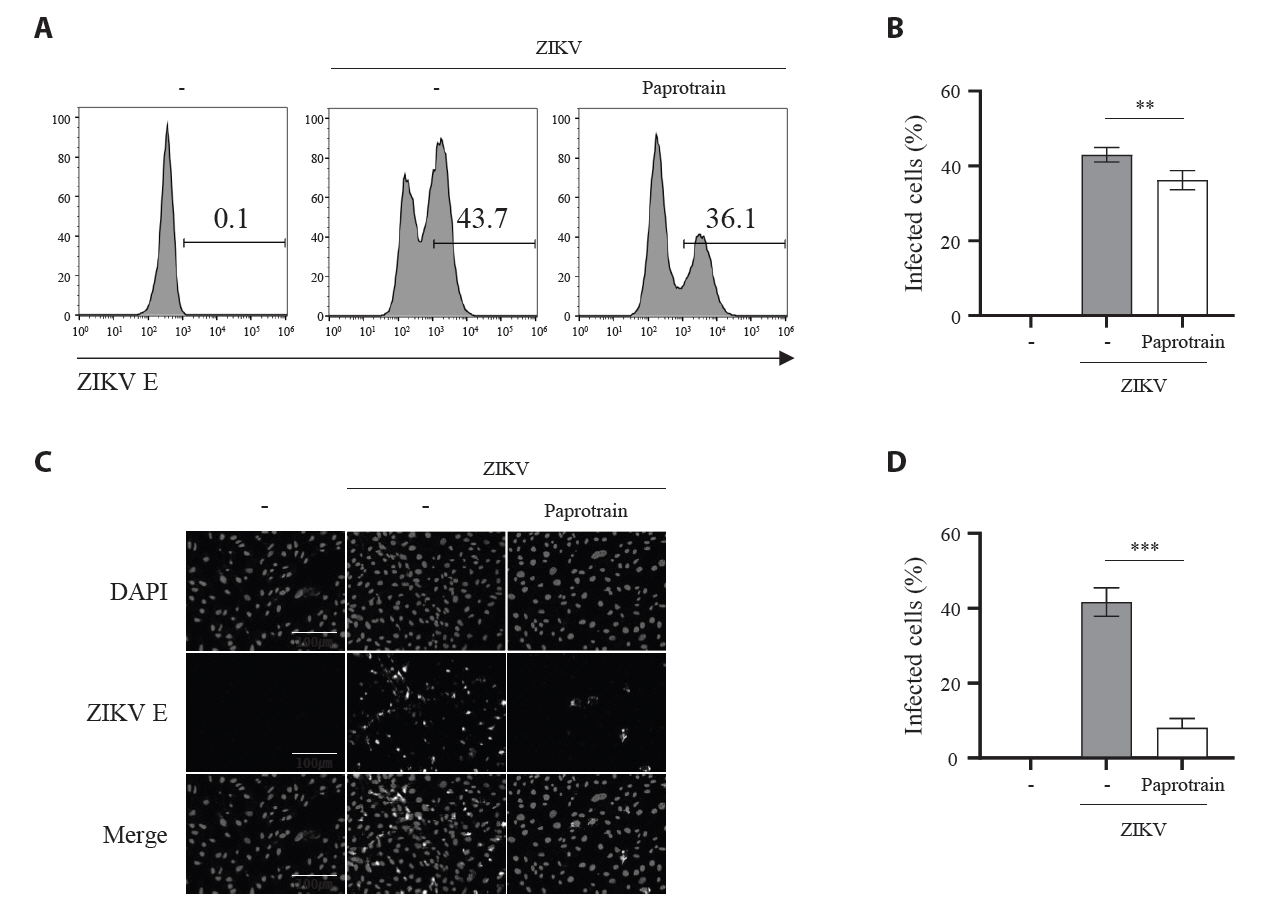
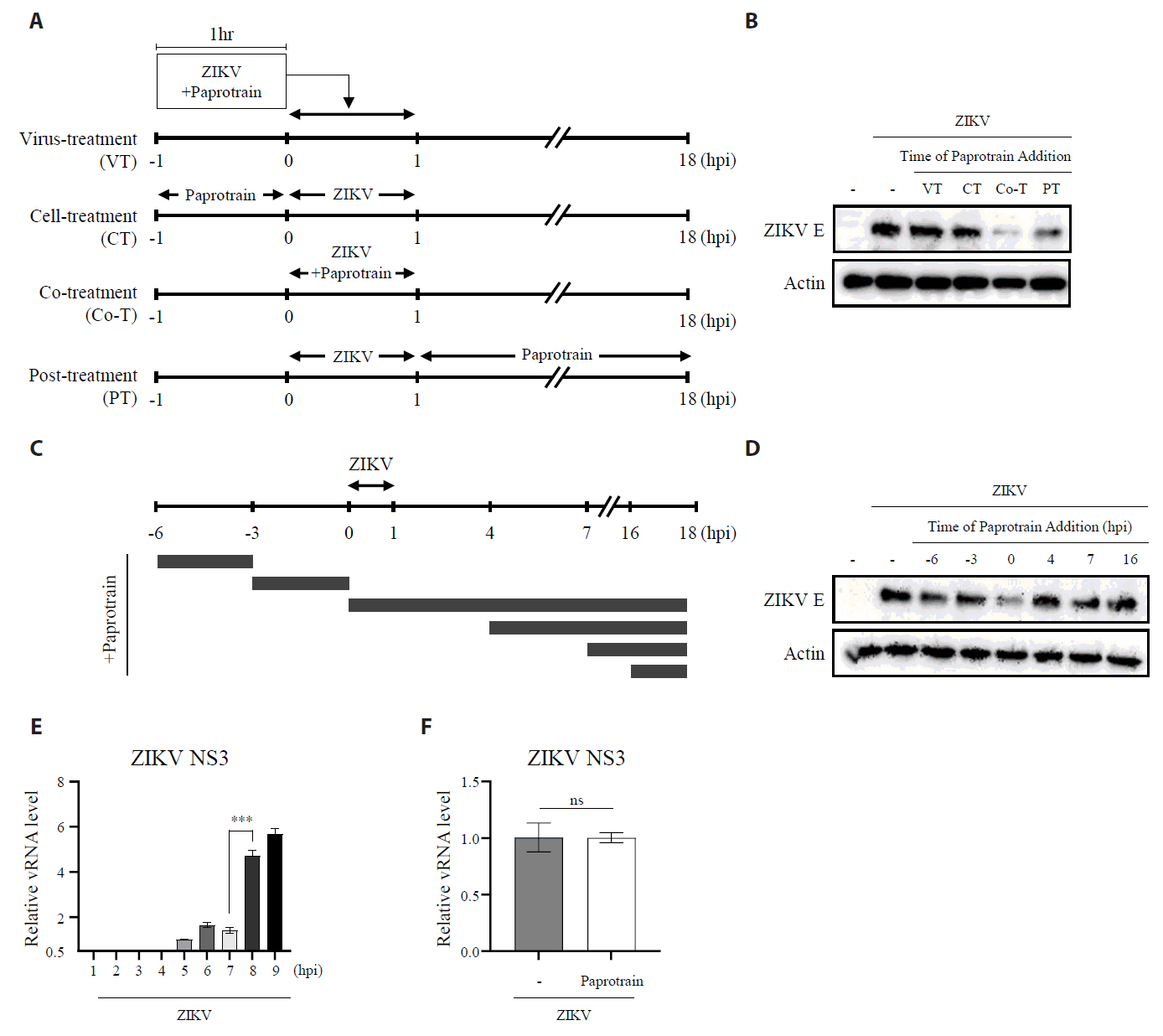
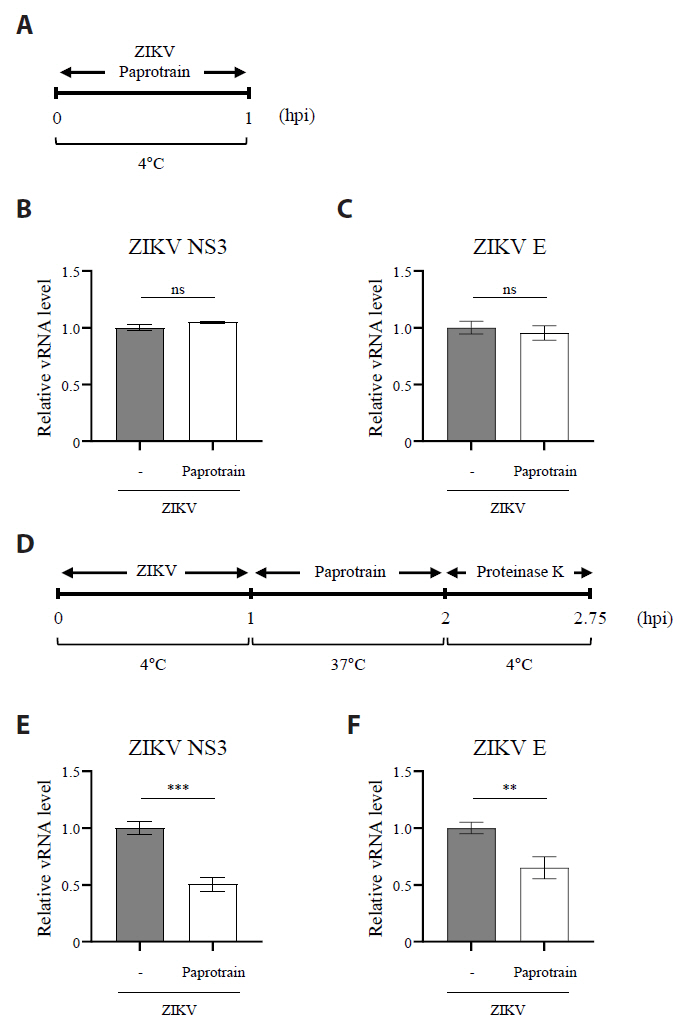
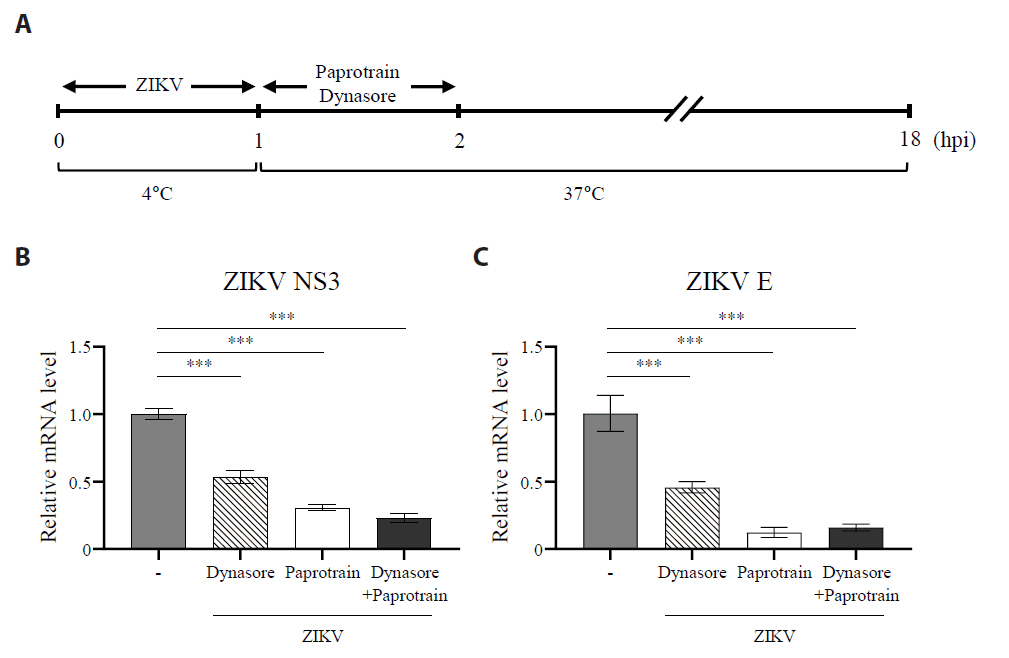
Fig. 1. Zika virus infection increases the expression of KIF20A. (A–C) Vero E6 cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 0.1 or 1. Cells were harvested at 6 h post-infection (hpi), and mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified using qRT-PCR. (A) Relative KIF20A mRNA levels normalized to GAPDH. (B, C) Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. (D–F) Vero E6 cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 1. Cells were harvested at 0, 2, 3, 4, 5, and 6 h post-infection, and mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified using qRT-PCR. (D) Relative KIF20A mRNA levels normalized to GAPDH. (E, F) Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Data are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
Fig. 2. KIF20A knockdown attenuates Zika virus infection. (A–C) Vero E6 cells were transfected with either control scrambled small interfering RNA (siRNA) or one of three different siRNA targeting KIF20A (siRNA #1, #2, and #3). After transfection, cells were infected with Zika virus (ZIKV) at a multiplicity of infection (MOI) of 5. At 6 h post-infection (hpi), total RNA was extracted, while the mRNA levels of KIF20A and ZIKV NS3 were quantified using qRT-PCR. (A) Relative KIF20A expressions normalized to GAPDH. (B) Relative mRNA levels of ZIKV E normalized to GAPDH. (C) KIF20A protein levels analyzed using western blot. (D–F) HEK293T cells were transduced with lentivirus encoding either control shRNA or KIF20A-targeting shRNA. Transduction efficiency was confirmed using a GFP reporter and selected with puromycin. Stable cell lines were infected with ZIKV at an MOI of 0.5. At 6 hpi, total RNA was extracted, while the mRNA levels of KIF20A, ZIKV NS3, and ZIKV E were quantified. (D) Relative mRNA levels of KIF20A normalized to GAPDH. (E, F) Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences using an as determined using an analysis of variance (ANOVA) (*P < 0.1, **P < 0.01, ***P < 0.001), or unpaired t-test (***P < 0.001).
Fig. 3. KIF20A inhibition suppresses Zika virus infection. (A) Vero E6 cells were treated with paprotrain at concentrations ranging from 100 to 1 µg/ml. After 12 h, cell viability was determined using the trypan blue exclusion assay. (B–E) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.5 and co-treated with either 20 µg/ml paprotrain or 50 µM chloroquine (CQ) for 18 h. (B, C) Relative mRNA levels of ZIKV NS3 (B) and E (C) normalized to GAPDH. (D) ZIKV E protein levels analyzed using Western blot. (E) Viral titers in the culture medium quantified using a focus-forming assay. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
Fig. 4. KIF20A inhibition reduces the number of ZIKV-infected cells. (A, B) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 5, with or without 20 µg/ml paprotrain. After 18 hpi, infected cells were fixed, permeabilized, and stained with an anti-ZIKV E protein antibody for flow cytometric analysis. (A) Representative histograms illustrating the proportion of ZIKV E–positive (infected) cells. (B) Percentages of infected cells. (C, D) Vero E6 cells infected with ZIKV at an MOI of 5, with or without 20 µg/ml paprotrain. After 18 hpi, infected cells were fixed, permeabilized, and stained with DAPI and an anti-ZIKV E protein antibody for immunofluorescence microscopy. (C) Representative images showing infected cells. (D) Quantification from three independent experiments, where infected cells were defined as the number of ZIKV E–positive nuclei (co-localized with DAPI) divided by the total number of DAPI–stained nuclei. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (*P < 0.01, ***P < 0.001).
Fig. 5. KIF20A inhibition attenuates Zika virus infection on host cells at an early time point. (A) Schematic representation of treatment conditions: virus treatment (VT), cell treatment (CT), co-treatment (Co-T), and post-treatment (PT). (B) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 0.5. At 18 h post-infection (hpi), cells were harvested and lysed, and ZIKV E protein levels were analyzed using Western blot. (C) Schematic diagram of the time-of addition assay. Cells were treated with 20 µg/ml paprotrain at various time points relative to ZIKV infection. For pretreatment, cells received paprotrain 6 h and 3 h before infection. For co-treatment and post-treatment, paprotrain was added at 0 h (co-treatment) or 4 h, 7 h, and 16 h post-infection. (D) Vero E6 cells were infected with ZIKV at an MOI of 0.5. At 18 hpi, cells were harvested and lysed, while ZIKV E protein levels were analyzed using Western blot. All experiments were performed at least twice. (E) Vero E6 cells were infected with ZIKV at an MOI of 1, and relative vRNA levels of ZIKV NS3 were normalized to GAPDH at each time point after ZIKV infection. (F) Vero E6 cells were infected with ZIKV at an MOI of 1, and treated with 20 µg/ml paprotrain at 7 hpi for 1 h. Relative vRNA levels of ZIKV NS3 were normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (***P < 0.001).
Fig. 6. The inhibition of KIF20A interferes with the Zika virus internalization during entry stages. (A) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV attachment. (B, C) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 10 with or without 20 µg/ml paprotrain at 4°C. After 1 h, cells were washed five times with cold PBS. The samples were then immediately processed for total RNA extraction. Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. (D) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV entry. (E, F) Vero E6 cells infected with ZIKV at a multiplicity of infection (MOI) of 10 for 1 h at 4°C. Following infection, cells were washed five times with cold PBS, followed by treatment with 20 µg/ml paprotrain. After incubation at 37°C for 1 h, the cells were washed with cold PBS and treated with proteinase K (1 mg/ml) for 45 min at 4°C to remove surface-bound but non-internalized virus. The samples were then immediately processed for total RNA extraction. Relative mRNA levels of ZIKV NS3 (E) and ZIKV E (F) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
Fig. 7. The inhibition of KIF20A suppresses clathrin-mediated entry of ZIKV. (A) Schematic representation of the experiment evaluating the effect of paprotrain on ZIKV clathrin-mediated endocytosis (CME) pathway. (B, C) Vero E6 cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.5 at 4°C. After 1 h, cells were washed three times with PBS and subsequently treated with paprotrain (20 µg/ml), dynasore (50 µM), or a combination of paprotrain (20 µg/ml) and dynasore (50 µM). At 18 h post infection (hpi), the levels of viral RNA were measured. Relative mRNA levels of ZIKV NS3 (B) and ZIKV E (C) normalized to GAPDH. Values are expressed as the mean ± standard error of the mean (SEM). All experiments were performed at least twice. Asterisks indicate statistically significant differences as determined using an analysis of variance (ANOVA) (**P < 0.01, ***P < 0.001).
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Inhibiting kinesin family member 20A disrupts Zika virus entry by blocking internalization
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article








