Articles
- Page Path
- HOME > J. Microbiol > Volume 63(9); 2025 > Article
-
Full article
Metal ion homeostasis regulates condensin-dependent chromatin architecture and chromosome segregation in Schizosaccharomyces pombe - Seong Ho An, Kyoung-Dong Kim*
-
Journal of Microbiology 2025;63(9):jm.2505008.
DOI: https://doi.org/10.71150/jm.2505008
Published online: August 29, 2025
Department of Systems Biotechnology, Chung-Ang University, Anseong 17546, Republic of Korea
- *Correspondence Kyoung-Dong Kim kdkim0122@cau.ac.kr
• Received: May 15, 2025 • Revised: June 19, 2025 • Accepted: June 20, 2025
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,843 Views
- 73 Download
ABSTRACT
- Condensin plays a central role in mitotic chromosome organization and segregation by mediating long-range chromatin interactions. However, the extent to which cellular metabolic status influences condensin function remains unclear. To gain insights into the relationship of metal ion homeostasis and the function of condensin, we conducted genome-wide chromatin immunoprecipitation sequencing (ChIP-seq) using Schizosaccharomyces pombe under iron- or zinc-deficient conditions. Under iron- or zinc-deficient conditions, ChIP-seq results revealed a selective reduction in condensin binding at high-affinity target loci, particularly genes regulated by Ace2 and Ams2, while cohesin binding remained largely unaffected. Hi-C analysis showed that iron depletion weakened chromatin interactions at these condensin targets and centromeres, without disrupting global genome architecture. DNA fluorescence in situ hybridization (FISH) confirmed that iron deficiency impaired long-range associations between centromeres and Ace2 target loci at the single-cell level. Notably, iron deficiency led to chromosome segregation defects during mitosis, suggesting that diminished condensin occupancy compromised genome stability. These changes occurred without significant alterations in condensin protein levels or global transcription, indicating a direct effect of metal ion availability on condensin activity. Collectively, our findings revealed a previously unrecognized regulatory axis in which cellular metal ion homeostasis modulated condensin-dependent chromatin organization and mitotic chromosome segregation, offering new insights into the integration of metabolic state with genome maintenance.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by Chung-Ang University Research Grants (2023) and a grant from the National Research Foundation of Korea (NRF) awarded to K.K. (2022R1A2C1004423).
Conflict of Interest
The authors declare no conflict of interest.
Ethical Statements
Not applicable.
Supplementary Information
Fig. 1.Misregulation of iron-uptake genes in the condensin mutant. (A) Transcript levels of iron-uptake genes in the condensin mutant (cut14-208). (B) Western blot showing depletion of Cut14 and Cut3 using the auxin-inducible degron (AID) system. 5-Adamantyl-IAA (100 nM) was used to induce degradation of AID-tagged proteins. (C) Chromosome segregation defects in the AID strain upon IAA treatment. The representative images show DAPI-stained cells exhibiting chromosome segregation defects following IAA treatment. Red arrows indicate cells with chromosomal segregation defects. More than 200 cells were analyzed. (D) Transcript levels of iron-uptake genes under condensin-depleted conditions using the AID system. Data represent the mean ± s.d. from three independent biological replicates and p values were calculated using a student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant).
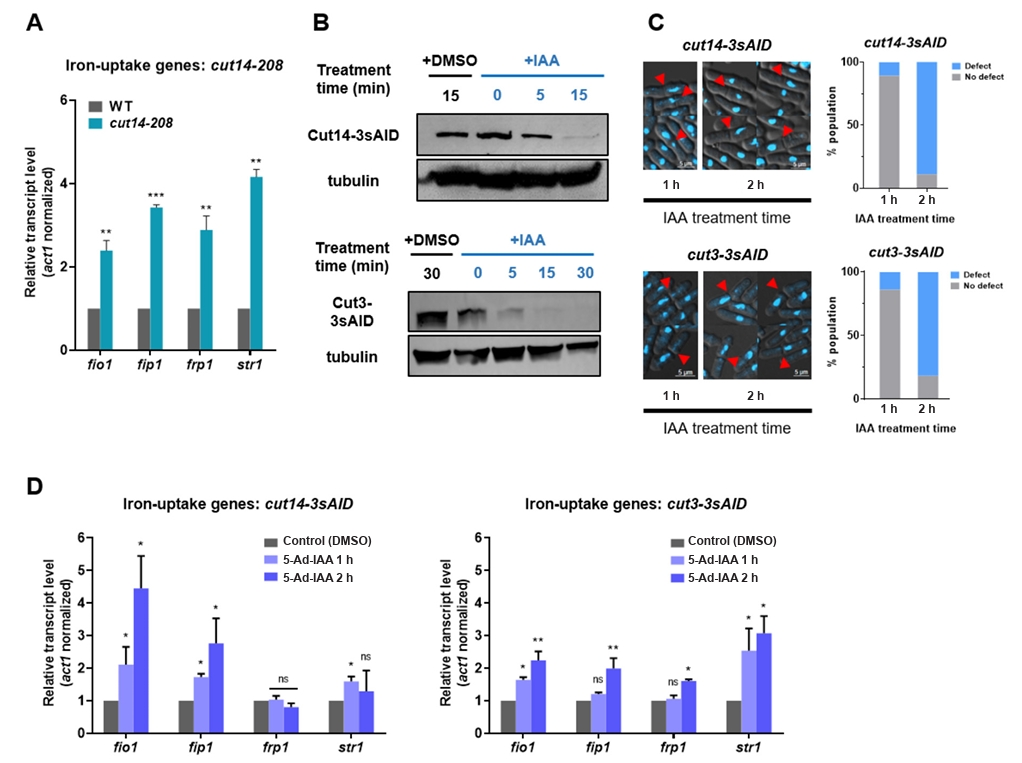

Fig. 2.Effects of iron depletion on S. pombe growth and chromosome stability. (A) Growth curves of S. pombe in the presence or absence of 250 µM DIP in the YES medium. (B) Scheme of cell-cycle synchronization using hydroxyurea (HU). (C) Chromosome segregation defects under iron-depleted conditions. The representative images show DAPI-stained cells exhibiting chromosome segregation defects following DIP treatment. Red arrows indicate cells with chromosomal segregation defects. The percentage of cells with segregation defects was quantified at the indicated time points (upper panel). Red arrows indicate segregation defects (lower panel). (D) Effect of iron depletion on mini-chromosome stability. Experiments were repeated twice. Total cells counted: n = 1,146 and 1,287 (iron-replete); n = 966 and 1,105 (iron-depleted). Statistical validation was performed based on a Mann-Whitney U test.
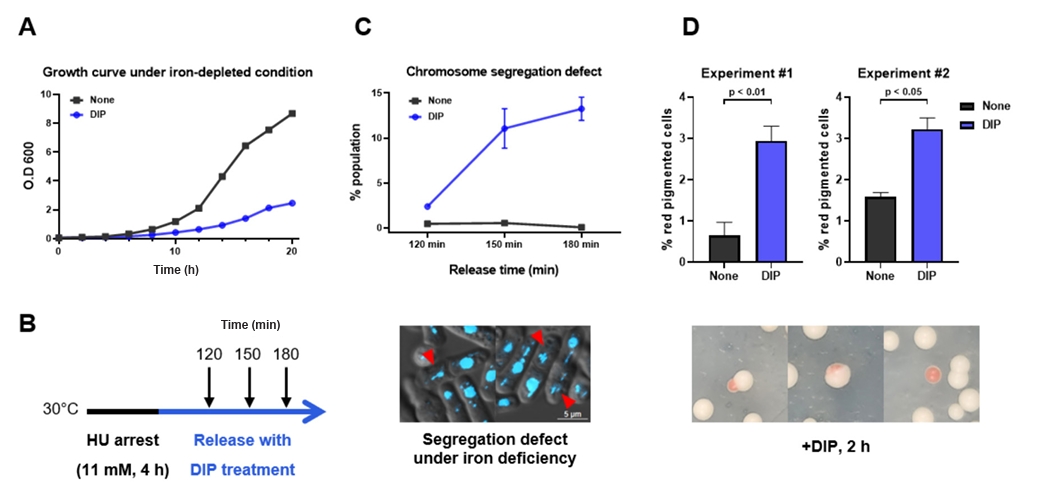

Fig. 3.Reduced condensin binding under iron-depleted conditions. (A) ChIP-seq profiles of Cut14-Pk binding across chromosomes under iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Some genes among the top 200 condensin binding sites are shown in black, and Ace2/Ams2 target genes are shown in green. (B) Condensin binding profiles at the top 200 high-affinity sites under both conditions. (C) Decreased condensin binding at condensin target genes under iron-depleted conditions. Line plots show enrichment at target loci. Each snap is enlarged image captured from Integrative Genome Viewer (IGV). (D) Box plot showing average Cut14-Pk enrichment across different gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (E) ChIP-qPCR analysis of Cut14-Pk enrichment at Ace2/Ams2 targets (adg1, adg3, htb1, and cfh4). Data represent the mean ± s.d. from three biological replicates and p values were calculated using a student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant).
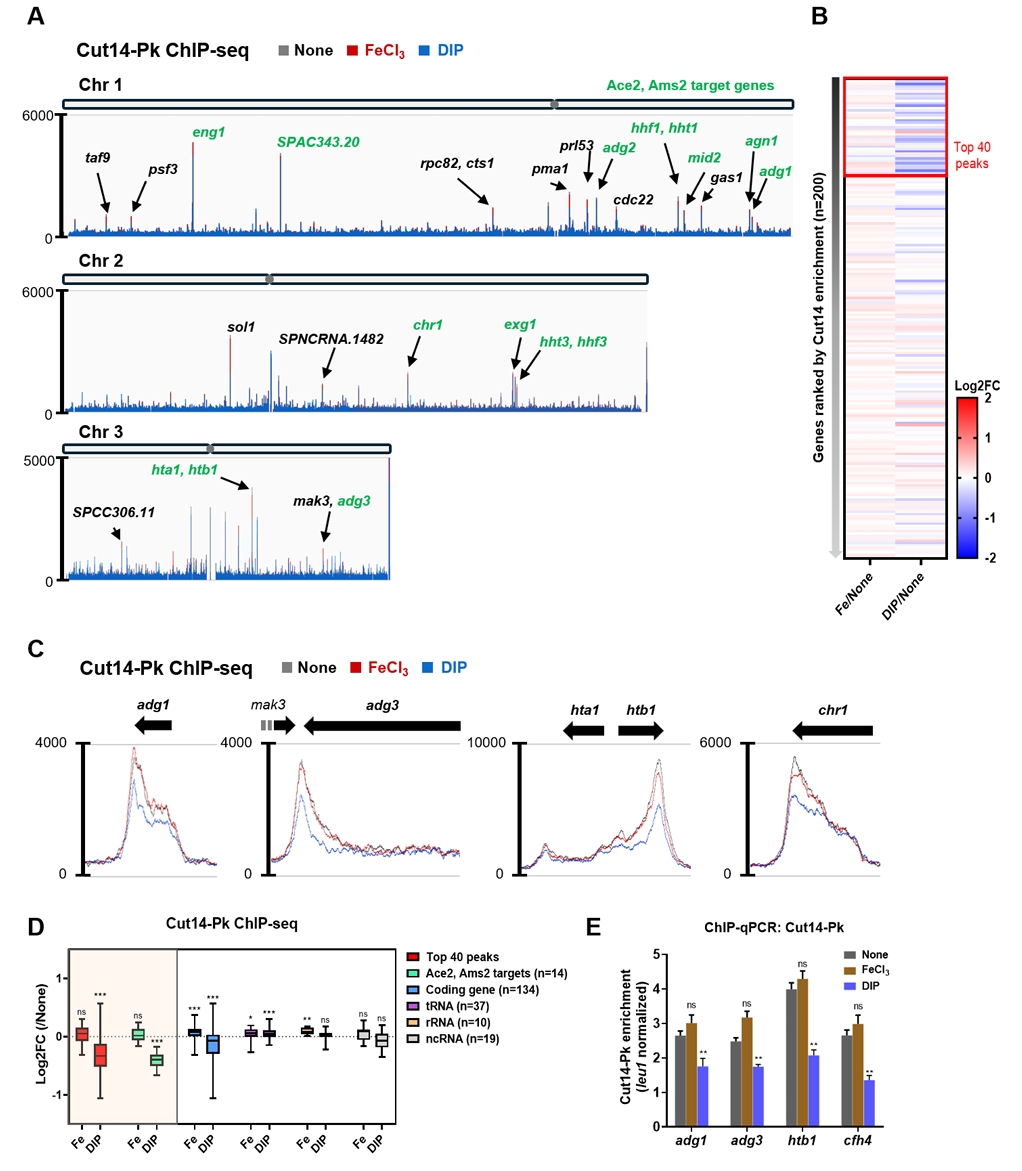

Fig. 4.Cohesin binding under iron-replete and iron-depleted conditions. (A) ChIP-seq profile of Rad21-Myc binding on chromosome I under both iron conditions. The top five cohesin-binding genes on chromosome I are shown in black. (B) Box plot showing average Rad21-Myc enrichment across gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Cohesin binding patterns at the top 200 high-affinity sites under iron-replete and iron-depleted conditions. (D) Rad21-Myc binding at tRNA and rRNA genes under different iron conditions. Line plots indicate enrichment under each condition. Each snap is enlarged image captured from IGV.
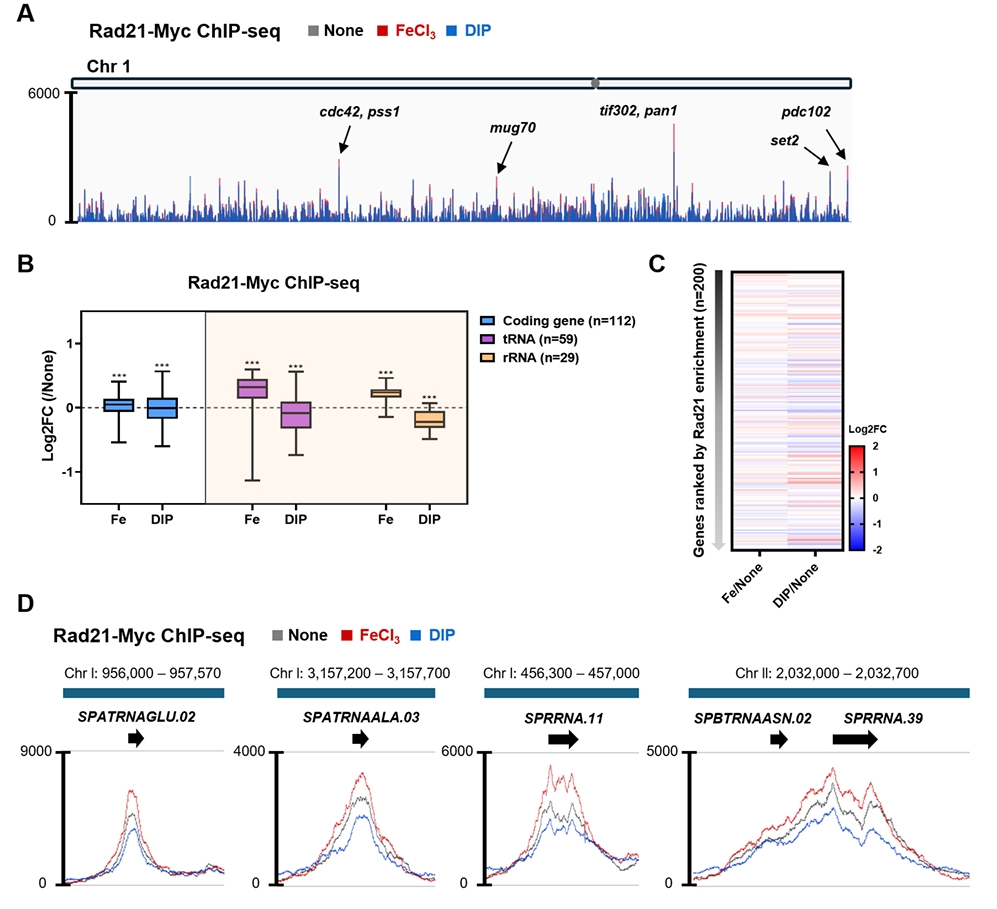

Fig. 5.Expression of SMC complex components and Ace2/Ams2 target genes under varying iron conditions. (A, B) Transcript levels of condensin subunits (A) and cohesin subunits (B) under iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Data represent the mean ± s.d. from three biological replicates and p values were calculated using student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Western blot showing protein levels of Cut14 and Cnd2 under different iron conditions. (D) Transcript levels of Ace2/Ams2 targets (eng1, htb1, hta2, cfh4, and hht3) under the same conditions. Data represent the mean ± s.d. from three biological replicates and p values were calculated using student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (E) Scatter with bar plot showing RNA polymerase II enrichment at genes across classes. Each dot on plot indicates individual gene value. (F) RNA polymerase II binding patterns at the top 200 high-affinity sites under iron-replete and iron-depleted conditions.
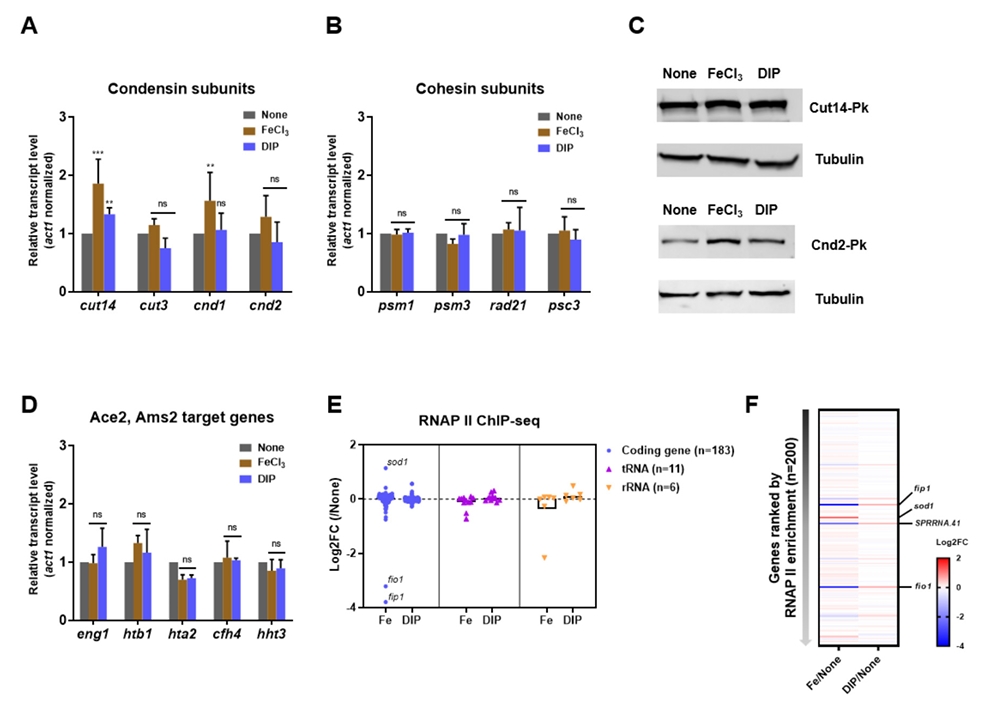

Fig. 6.Genome organization under iron-replete and iron-depleted conditions. (A) Hi-C differential contact map (10-kb binning) for chromosome I between iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Regions with altered contacts are outlined with white dashed boxes. (B) Differential contact map and ChIP-seq enrichment profiles of Cut14-Pk (left square in A; chromosome I: 0.6-1.1 Mb). (C) Differential contact map of centromeric region (middle square in A; chromosome I: 3.5–4.0 Mb). (D) Differential contact map and ChIP-seq enrichment of Cut14-Pk for the right square in A (chromosome I: 4.22–4.25 Mb), encompassing iron-uptake genes fip1 and fio1.
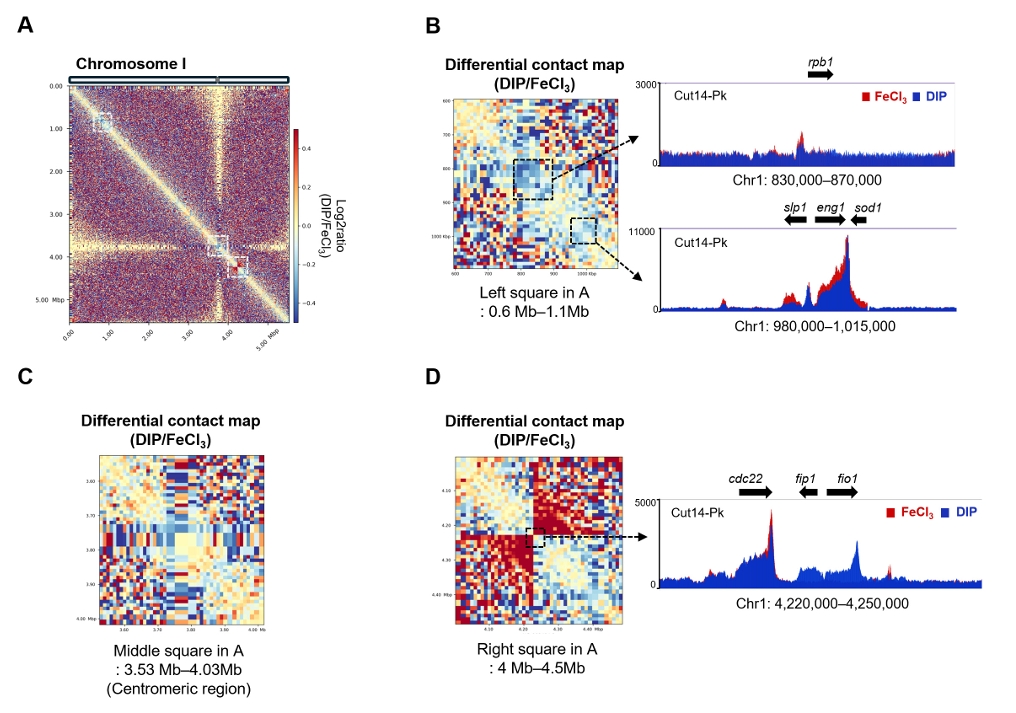

Fig. 7.Reduced condensin-mediated chromosomal associations under iron-depleted conditions. (A) Genomic positions of FISH probes targeting Cut14-Pk-enriched loci near eng1 and adg1, and a negative control (SPAC110). ChIP-seq signals confirm condensin binding at eng1 and adg1 but not at the control locus. (B, C) FISH analysis of inter-locus distances between eng1 and centromere I (cnt1), or eng1 and adg1, under iron-replete and iron-depleted conditions. Representative images are shown above plots. Distances were measured in > 100 cells and binned. Statistical validation was performed based on a Mann-Whitney U test.
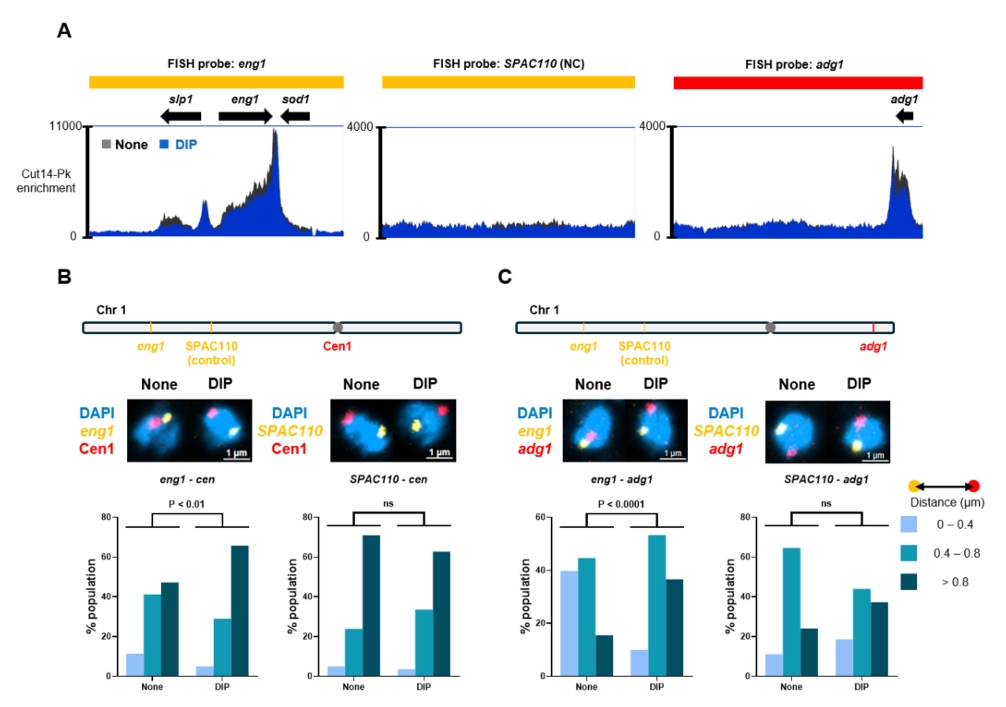

Fig. 8.Condensin binding patterns under zinc-depleted conditions. (A) ChIP-seq profiles of Cut14-Pk binding on chromosome I under zinc-depleted conditions (50 µM and 100 µM TPEN). Ace2/Ams2 targets are shown in green. (B) Box plot showing average Cut14-Pk enrichment across gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Condensin binding profiles at the top 200 high-affinity sites under zinc-depleted conditions. (D) Reduced condensin binding at target genes under zinc depletion (100 µM TPEN). Bar plots show enrichment levels at these loci. Each snap is enlarged image captured from IGV. (E) Graphical abstract of this study. This figure created with BioRender (https://BioRender.com).

- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9: 218–233. ArticlePubMed
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie III A, et al. 1998. Heterologous modules for efficient and versatile PCR‐based gene targeting in Schizosaccharomyces pombe. Yeast. 14: 943–951. ArticlePubMed
- Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, et al. 1989. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell. 57: 739–751. ArticlePubMed
- Chowdhary S, Kainth AS, Paracha S, Gross DS, Pincus D. 2022. Inducible transcriptional condensates drive 3D genome reorganization in the heat shock response. Mol Cell. 82: 4386–4399. ArticlePubMedPMC
- Corkins ME, May M, Ehrensberger KM, Hu Y-M, Liu Y-H, et al. 2013. Zinc finger protein Loz1 is required for zinc-responsive regulation of gene expression in fission yeast. Proc Natl Acad Sci USA. 110: 15371–15376. ArticlePubMedPMC
- Gadaleta MC, Iwasaki O, Noguchi C, Noma K-I, Noguchi E. 2015. Chromatin immunoprecipitation to detect DNA replication and repair factors. Methods Mol Biol. 1300: 169–186. ArticlePubMedPMC
- Gallagher PS, Larkin M, Thillainadesan G, Dhakshnamoorthy J, Balachandran V, et al. 2018. Iron homeostasis regulates facultative heterochromatin assembly in adaptive genome control. Nat Struct Mol Biol. 25: 372–383. ArticlePubMedPMCPDF
- Hirano T. 2012. Condensins: Universal organizers of chromosomes with diverse functions. Genes Dev. 26: 1659–1678. ArticlePubMedPMC
- Holtzen SE, Navid E, Kainov JD, Palmer AE. 2024. Transient Zn2+ deficiency induces replication stress and compromises daughter cell proliferation. Proc Natl Acad Sci USA. 121: e2321216121. ArticlePubMedPMC
- Iwasaki O, Tanaka A, Tanizawa H, Grewal SI, Noma KI. 2010. Centromeric localization of dispersed Pol III genes in fission yeast. Mol Biol Cell. 21: 254–265. ArticlePubMedPMC
- Kakui Y, Rabinowitz A, Barry DJ, Uhlmann F. 2017. Condensin-mediated remodeling of the mitotic chromatin landscape in fission yeast. Nat Genet. 49: 1553–1557. ArticlePubMedPMCPDF
- Killilea DW, Ames BN. 2008. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci USA. 105: 5768–5773. ArticlePubMedPMC
- Kim K-D, Iwasaki O, Noma K. 2016a. An IF-FISH approach for covisualization of gene loci and nuclear architecture in fission yeast. Methods Enzymol. 574: 167–180. ArticlePubMedPMC
- Kim K-D, Tanizawa H, Iwasaki O, Noma K-i. 2016b. Transcription factors mediate condensin recruitment and global chromosomal organization in fission yeast. Nat Genet. 48: 1242–1252. ArticlePubMedPMCPDF
- Klug A. 2010. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 79: 213–231. ArticlePubMed
- Labbé S, Pelletier B, Mercier A. 2007. Iron homeostasis in the fission yeast Schizosaccharomyces pombe. Biometals. 20: 523–537. ArticlePubMed
- Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, et al. 2004. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 430: 573–578. ArticlePubMedPMCPDF
- Li L, Lyu X, Hou C, Takenaka N, Nguyen HQ, et al. 2015. Widespread rearrangement of 3D chromatin organization underlies polycomb-mediated stress-induced silencing. Mol Cell. 58: 216–231. ArticlePubMedPMC
- Mizuguchi T, Fudenberg G, Mehta S, Belton JM, Taneja N, et al. 2014. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. Pombe. Nature. 516: 432–435. ArticlePubMedPMCPDF
- Muckenthaler MU, Rivella S, Hentze MW, Galy B. 2017. A red carpet for iron metabolism. Cell. 168: 344–361. ArticlePubMedPMC
- Nakazawa N, Arakawa O, Yanagida M. 2019. Condensin locates at transcriptional termination sites in mitosis, possibly releasing mitotic transcripts. Open Biol. 9: 190125.ArticlePubMedPMCPDF
- Nasmyth K, Haering CH. 2009. Cohesin: Its roles and mechanisms. Annu Rev Genet. 43: 525–558. ArticlePubMed
- Niwa O. 2018. Determination of the frequency of minichromosome loss to assess chromosome stability in fission yeast. Cold Spring Harb Protoc. 2018: pdb.prot091991.ArticlePubMed
- Ocampo D, Damon LJ, Sanford L, Holtzen SE, Jones T, et al. 2024. Cellular zinc status alters chromatin accessibility and binding of p53 to DNA. Life Sci Alliance. 7: e202302327. ArticlePubMedPMC
- Ono T, Fang Y, Spector DL, Hirano T. 2004. Spatial and temporal regulation of condensins I and II in mitotic chromosome assembly in human cells. Mol Biol Cell. 15: 3296–3308. ArticlePubMedPMC
- Pelletier B, Trott A, Morano KA, Labbé S. 2005. Functional characterization of the iron-regulatory transcription factor Fep1 from Schizosaccharomyces pombe. J Biol Chem. 280: 25146–25161. ArticlePubMed
- Qin Y, Grimm SA, Roberts JD, Chrysovergis K, Wade PA. 2020. Alterations in promoter interaction landscape and transcriptional network underlying metabolic adaptation to diet. Nat Commun. 11: 962.ArticlePubMedPMCPDF
- Sissi C, Palumbo M. 2009. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 37: 702–711. ArticlePubMedPMC
- Sutani T, Sakata T, Nakato R, Masuda K, Ishibashi M, et al. 2015. Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation. Nat Commun. 6: 7815.ArticlePubMedPMCPDF
- Tanizawa H, Kim K-D, Iwasaki O, Noma K-i. 2017. Architectural alterations of the fission yeast genome during the cell cycle. Nat Struct Mol Biol. 24: 965–976. ArticlePubMedPMCPDF
- Wilson S, Liu YH, Cardona-Soto C, Wadhwa V, Foster MP, et al. 2019. The Loz1 transcription factor from Schizosaccharomyces pombe binds to Loz1 response elements and represses gene expression when zinc is in excess. Mol Microbiol. 112: 1701–1717. ArticlePubMedPMCPDF
- Yan M, Song Y, Wong CP, Hardin K, Ho E. 2008. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr. 138: 667–673. ArticlePubMed
- Zhang X-R, Zhao L, Suo F, Gao Y, Wu Q, et al. 2022. An improved auxin-inducible degron system for fission yeast. G3 (Bethesda). 12: jkab393.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations
Citations to this article as recorded by 

Metal ion homeostasis regulates condensin-dependent chromatin architecture and chromosome segregation in Schizosaccharomyces pombe
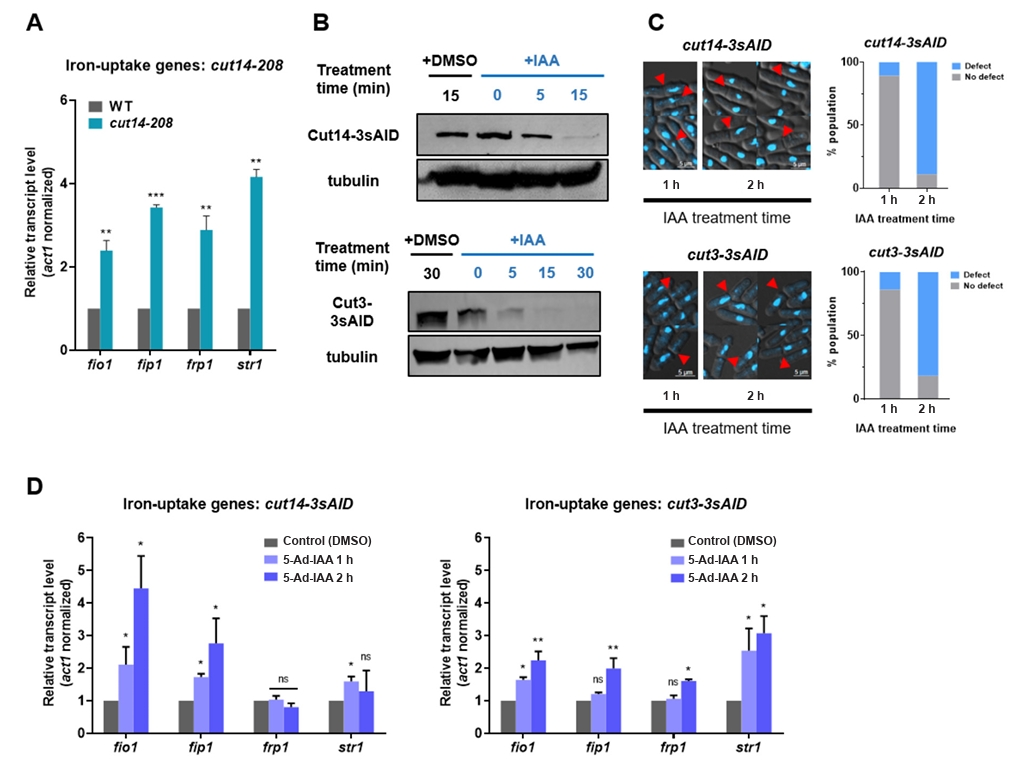
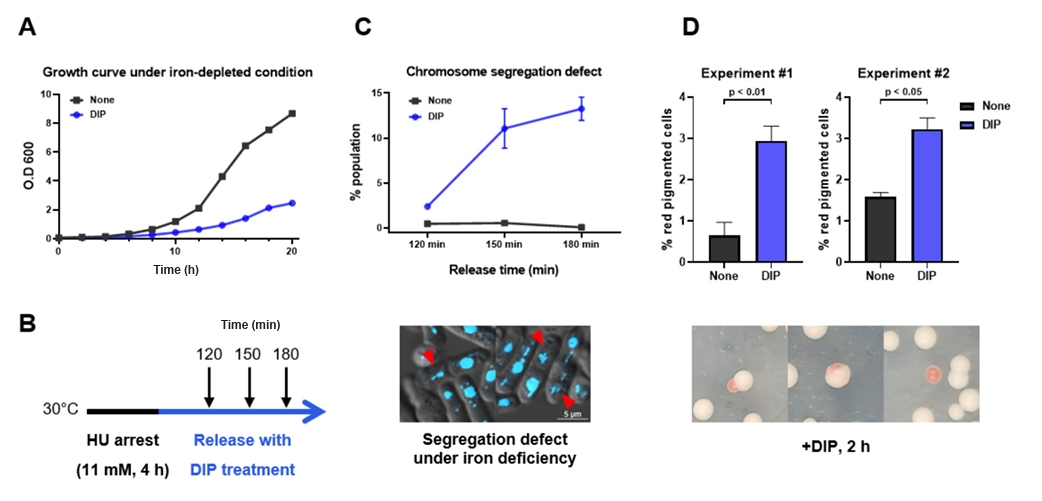
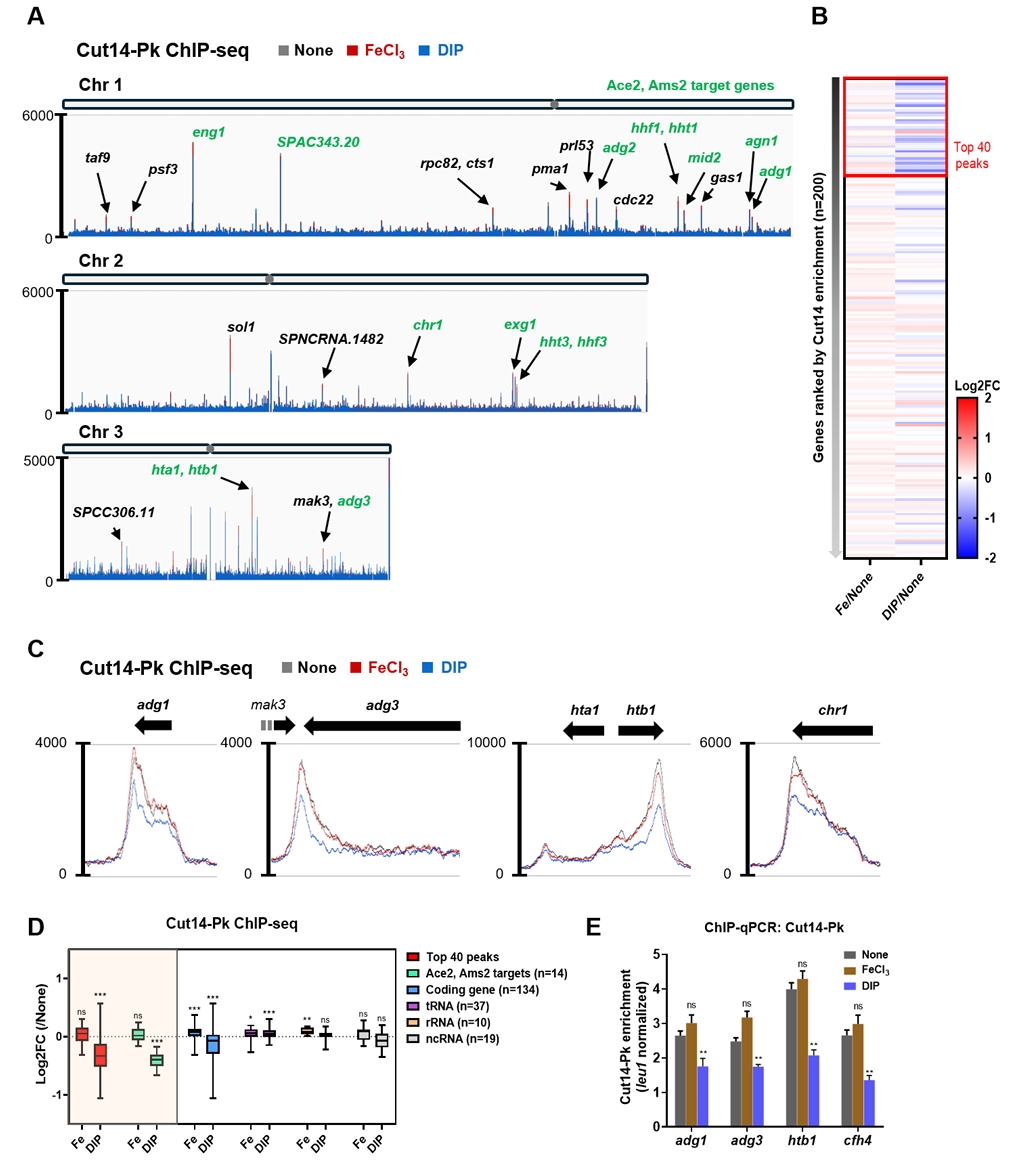
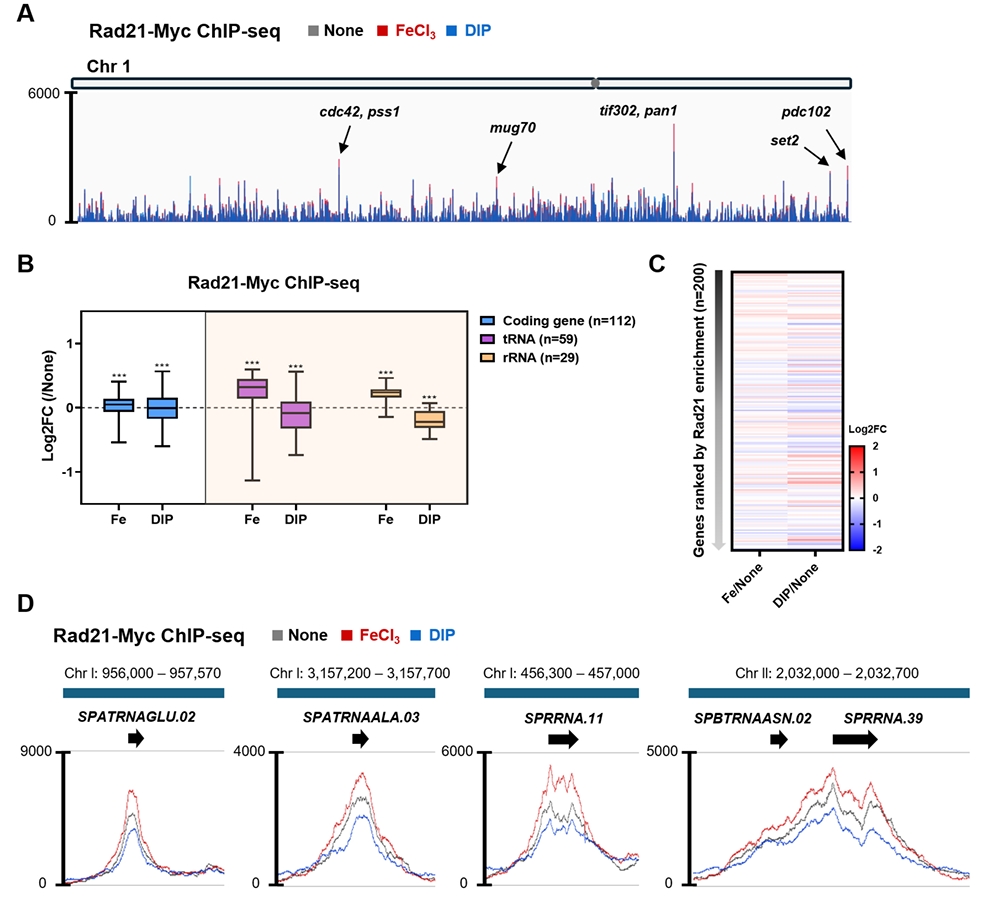
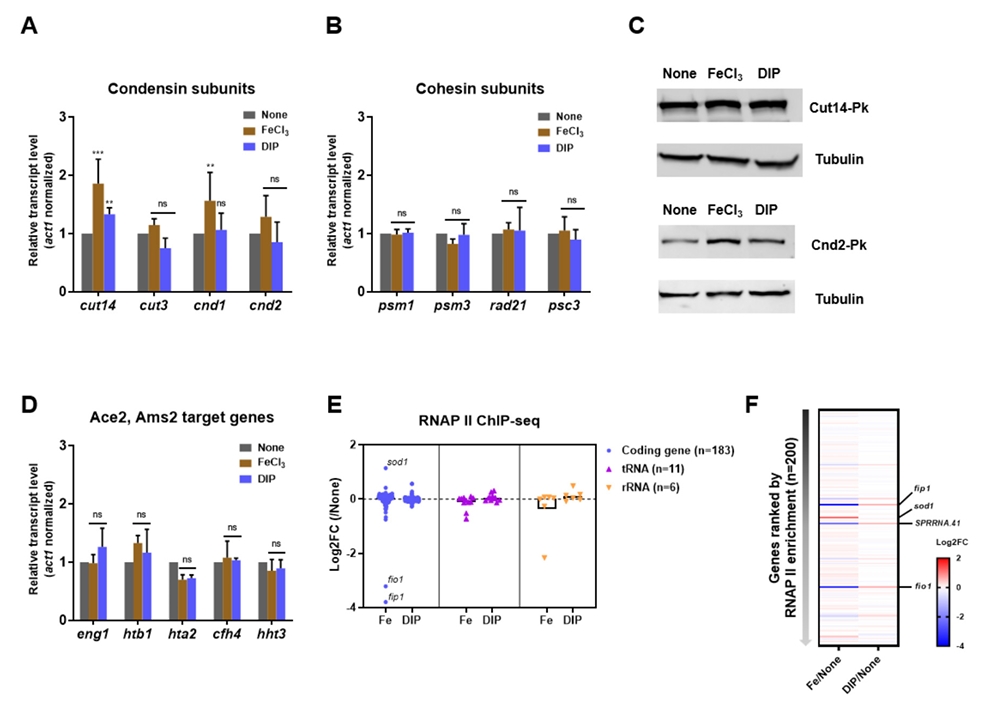
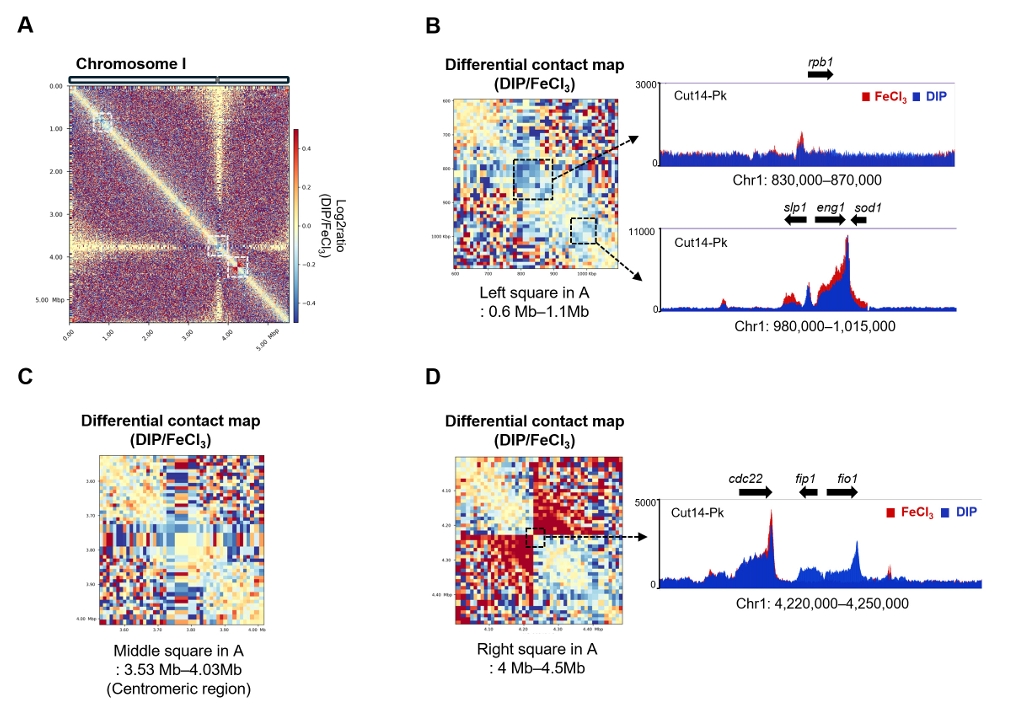
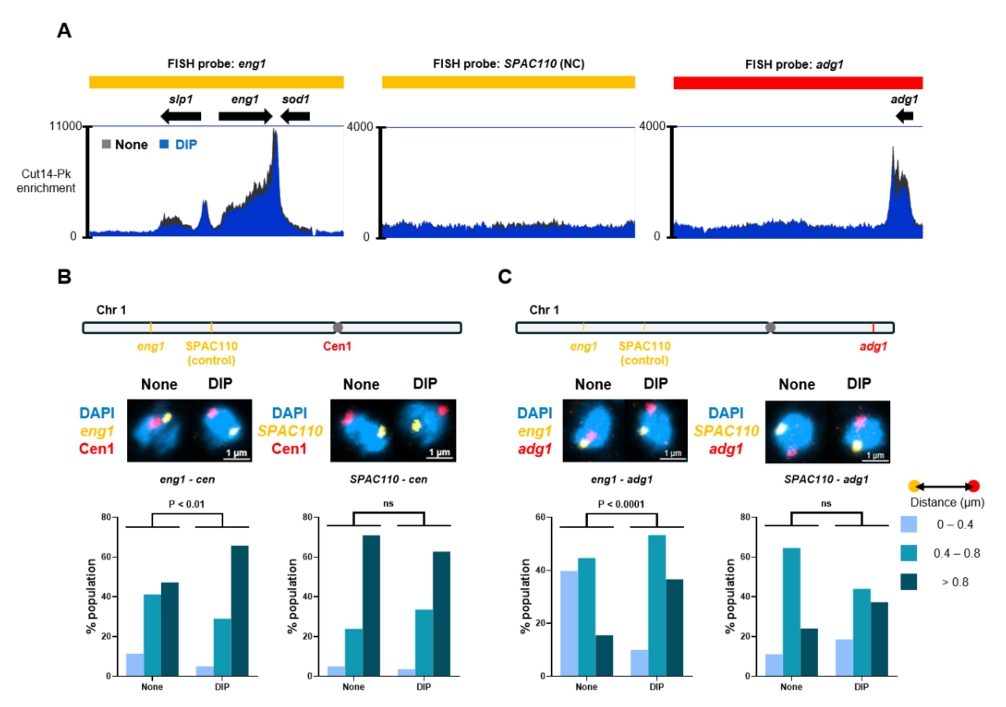
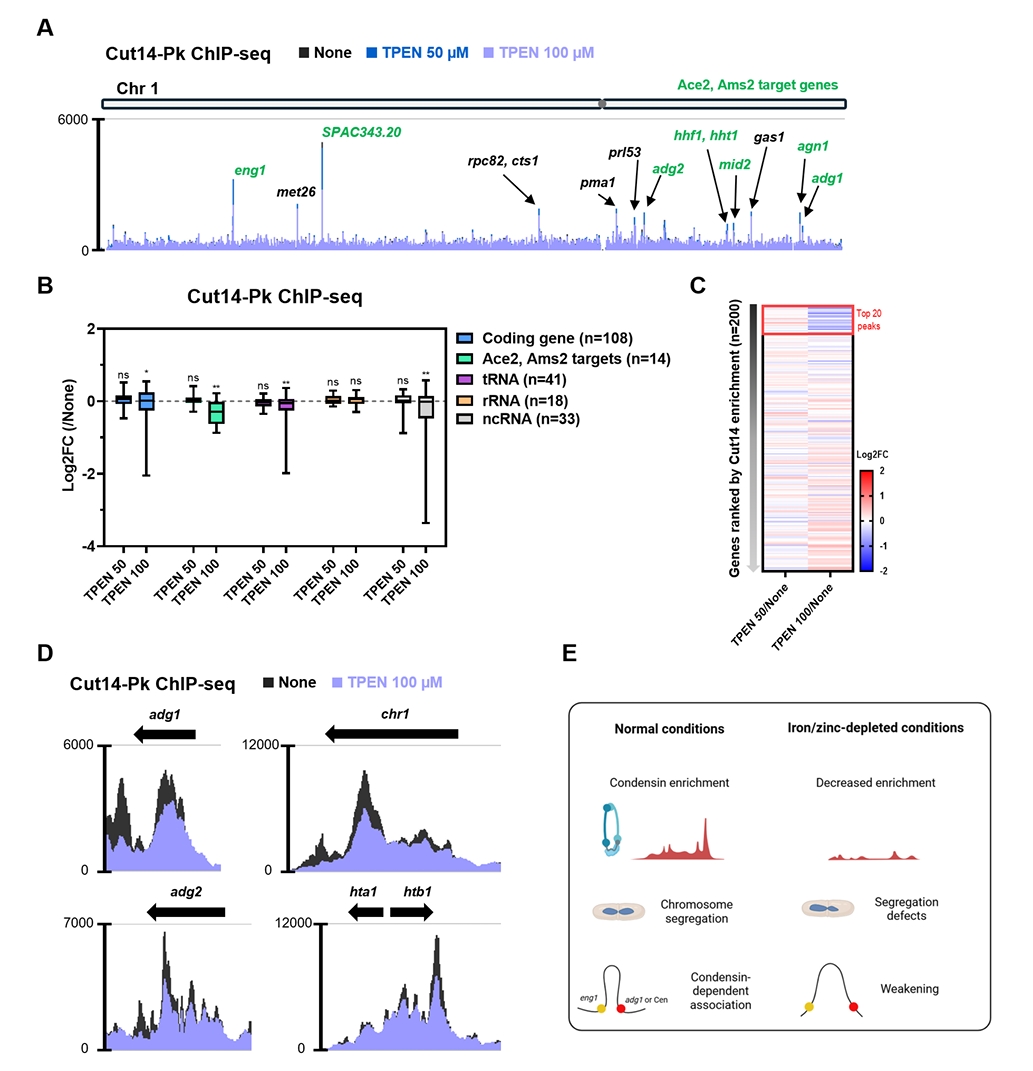
Fig. 1. Misregulation of iron-uptake genes in the condensin mutant. (A) Transcript levels of iron-uptake genes in the condensin mutant (cut14-208). (B) Western blot showing depletion of Cut14 and Cut3 using the auxin-inducible degron (AID) system. 5-Adamantyl-IAA (100 nM) was used to induce degradation of AID-tagged proteins. (C) Chromosome segregation defects in the AID strain upon IAA treatment. The representative images show DAPI-stained cells exhibiting chromosome segregation defects following IAA treatment. Red arrows indicate cells with chromosomal segregation defects. More than 200 cells were analyzed. (D) Transcript levels of iron-uptake genes under condensin-depleted conditions using the AID system. Data represent the mean ± s.d. from three independent biological replicates and p values were calculated using a student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant).
Fig. 2. Effects of iron depletion on S. pombe growth and chromosome stability. (A) Growth curves of S. pombe in the presence or absence of 250 µM DIP in the YES medium. (B) Scheme of cell-cycle synchronization using hydroxyurea (HU). (C) Chromosome segregation defects under iron-depleted conditions. The representative images show DAPI-stained cells exhibiting chromosome segregation defects following DIP treatment. Red arrows indicate cells with chromosomal segregation defects. The percentage of cells with segregation defects was quantified at the indicated time points (upper panel). Red arrows indicate segregation defects (lower panel). (D) Effect of iron depletion on mini-chromosome stability. Experiments were repeated twice. Total cells counted: n = 1,146 and 1,287 (iron-replete); n = 966 and 1,105 (iron-depleted). Statistical validation was performed based on a Mann-Whitney U test.
Fig. 3. Reduced condensin binding under iron-depleted conditions. (A) ChIP-seq profiles of Cut14-Pk binding across chromosomes under iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Some genes among the top 200 condensin binding sites are shown in black, and Ace2/Ams2 target genes are shown in green. (B) Condensin binding profiles at the top 200 high-affinity sites under both conditions. (C) Decreased condensin binding at condensin target genes under iron-depleted conditions. Line plots show enrichment at target loci. Each snap is enlarged image captured from Integrative Genome Viewer (IGV). (D) Box plot showing average Cut14-Pk enrichment across different gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (E) ChIP-qPCR analysis of Cut14-Pk enrichment at Ace2/Ams2 targets (adg1, adg3, htb1, and cfh4). Data represent the mean ± s.d. from three biological replicates and p values were calculated using a student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant).
Fig. 4. Cohesin binding under iron-replete and iron-depleted conditions. (A) ChIP-seq profile of Rad21-Myc binding on chromosome I under both iron conditions. The top five cohesin-binding genes on chromosome I are shown in black. (B) Box plot showing average Rad21-Myc enrichment across gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Cohesin binding patterns at the top 200 high-affinity sites under iron-replete and iron-depleted conditions. (D) Rad21-Myc binding at tRNA and rRNA genes under different iron conditions. Line plots indicate enrichment under each condition. Each snap is enlarged image captured from IGV.
Fig. 5. Expression of SMC complex components and Ace2/Ams2 target genes under varying iron conditions. (A, B) Transcript levels of condensin subunits (A) and cohesin subunits (B) under iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Data represent the mean ± s.d. from three biological replicates and p values were calculated using student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Western blot showing protein levels of Cut14 and Cnd2 under different iron conditions. (D) Transcript levels of Ace2/Ams2 targets (eng1, htb1, hta2, cfh4, and hht3) under the same conditions. Data represent the mean ± s.d. from three biological replicates and p values were calculated using student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (E) Scatter with bar plot showing RNA polymerase II enrichment at genes across classes. Each dot on plot indicates individual gene value. (F) RNA polymerase II binding patterns at the top 200 high-affinity sites under iron-replete and iron-depleted conditions.
Fig. 6. Genome organization under iron-replete and iron-depleted conditions. (A) Hi-C differential contact map (10-kb binning) for chromosome I between iron-replete (100 µM FeCl3) and iron-depleted (250 µM DIP) conditions. Regions with altered contacts are outlined with white dashed boxes. (B) Differential contact map and ChIP-seq enrichment profiles of Cut14-Pk (left square in A; chromosome I: 0.6-1.1 Mb). (C) Differential contact map of centromeric region (middle square in A; chromosome I: 3.5–4.0 Mb). (D) Differential contact map and ChIP-seq enrichment of Cut14-Pk for the right square in A (chromosome I: 4.22–4.25 Mb), encompassing iron-uptake genes fip1 and fio1.
Fig. 7. Reduced condensin-mediated chromosomal associations under iron-depleted conditions. (A) Genomic positions of FISH probes targeting Cut14-Pk-enriched loci near eng1 and adg1, and a negative control (SPAC110). ChIP-seq signals confirm condensin binding at eng1 and adg1 but not at the control locus. (B, C) FISH analysis of inter-locus distances between eng1 and centromere I (cnt1), or eng1 and adg1, under iron-replete and iron-depleted conditions. Representative images are shown above plots. Distances were measured in > 100 cells and binned. Statistical validation was performed based on a Mann-Whitney U test.
Fig. 8. Condensin binding patterns under zinc-depleted conditions. (A) ChIP-seq profiles of Cut14-Pk binding on chromosome I under zinc-depleted conditions (50 µM and 100 µM TPEN). Ace2/Ams2 targets are shown in green. (B) Box plot showing average Cut14-Pk enrichment across gene classes. p values were calculated using one sample t-test (***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant). (C) Condensin binding profiles at the top 200 high-affinity sites under zinc-depleted conditions. (D) Reduced condensin binding at target genes under zinc depletion (100 µM TPEN). Bar plots show enrichment levels at these loci. Each snap is enlarged image captured from IGV. (E) Graphical abstract of this study. This figure created with BioRender (https://BioRender.com).
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Metal ion homeostasis regulates condensin-dependent chromatin architecture and chromosome segregation in Schizosaccharomyces pombe
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article









