Articles
- Page Path
- HOME > J. Microbiol > Volume 63(9); 2025 > Article
-
Full article
PhoU interaction with the PhoR PAS domain is required for repression of the pho regulon and Salmonella virulence, but not for polyphosphate accumulation - Seungwoo Baek1,†, Soomin Choi1,†, Yoontak Han1,†, Eunna Choi1, Shinae Park2, Jung-Shin Lee2, Eun-Jin Lee1,*
-
Journal of Microbiology 2025;63(9):e2505013.
DOI: https://doi.org/10.71150/jm.2505013
Published online: September 30, 2025
1Department of Life Sciences, School of Life Sciences and Biotechnology, Korea University, Seoul 02841, Republic of Korea
2Department of Molecular Bioscience, College of Biomedical Science, Kangwon National University, Chuncheon 24341, Republic of Korea
- *Correspondence Eun-Jin Lee eunjinlee@korea.ac.kr
- †These authors contributed equally to this work
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,762 Views
- 46 Download
ABSTRACT
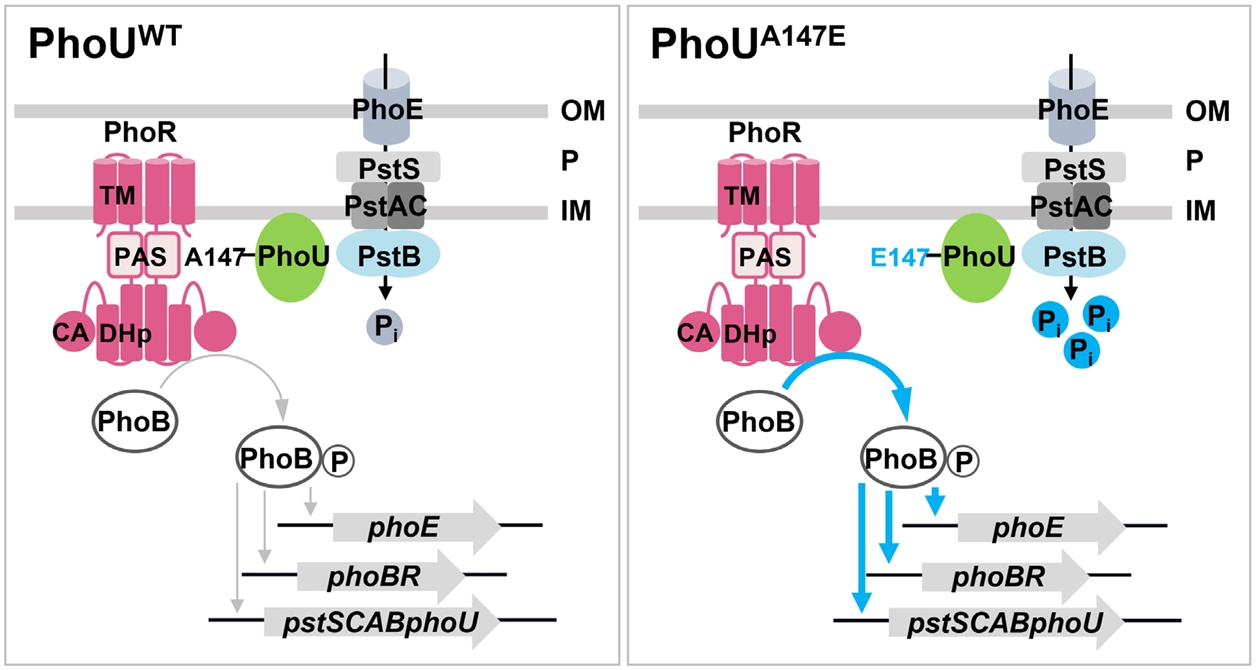
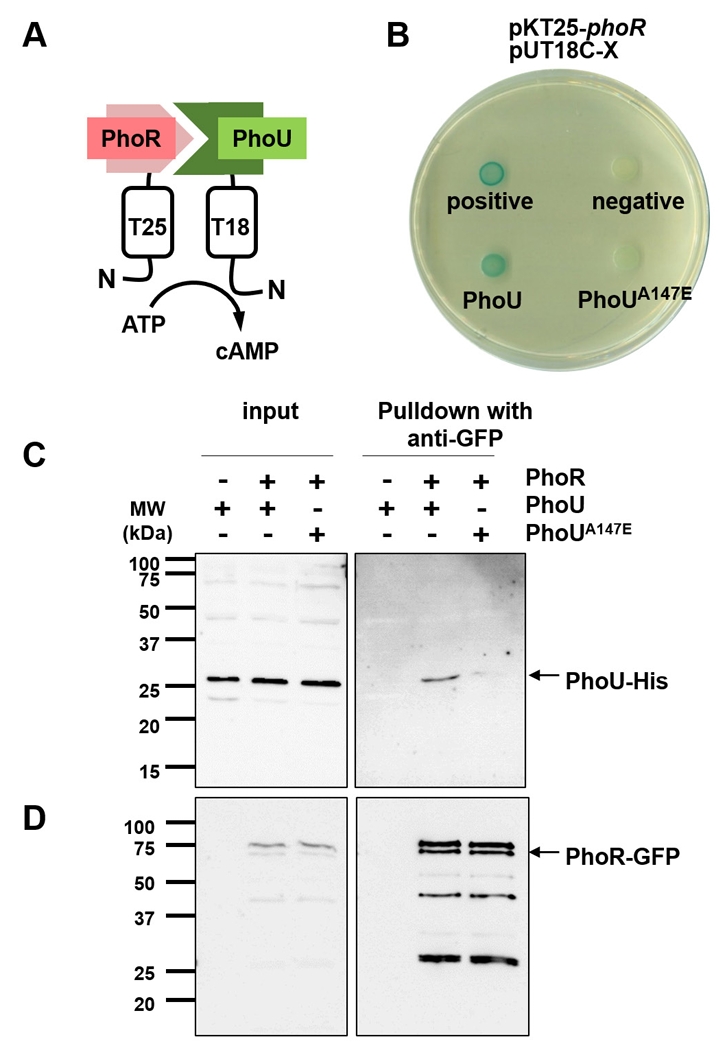
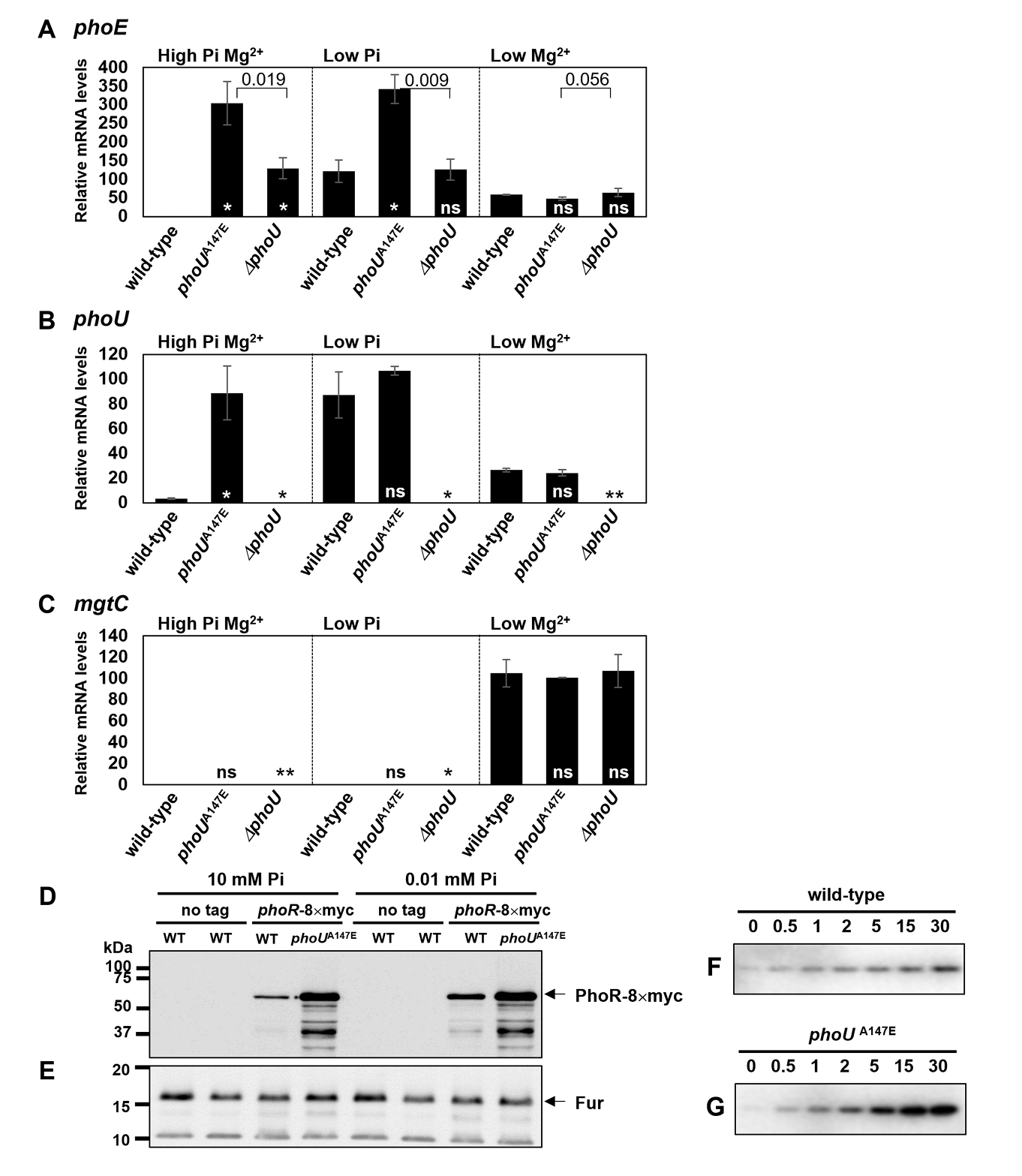
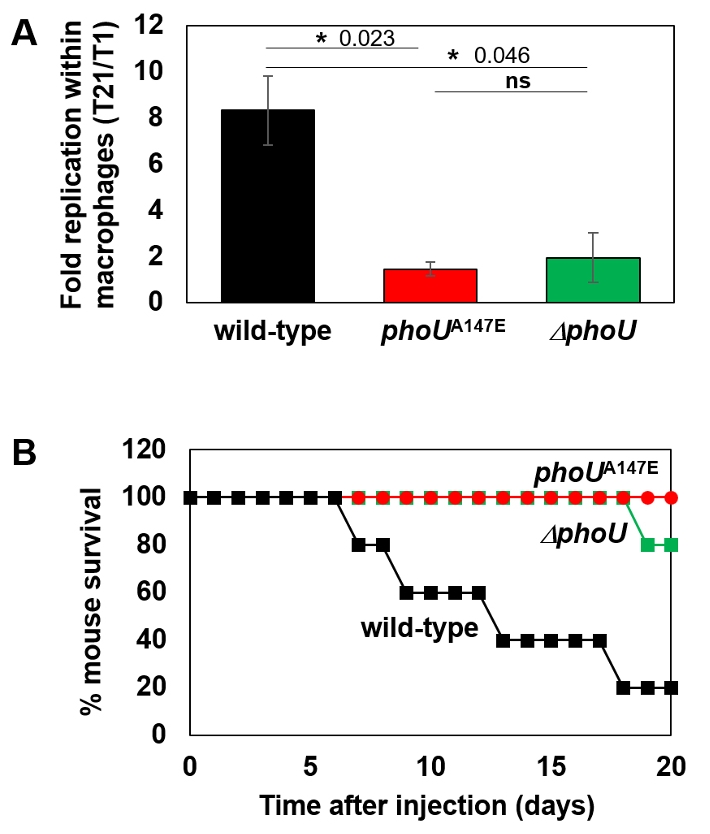
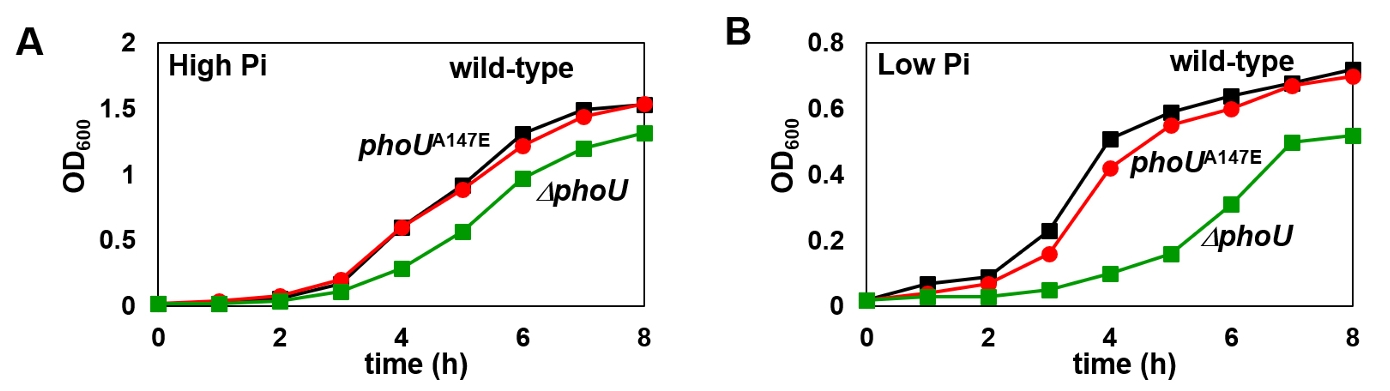
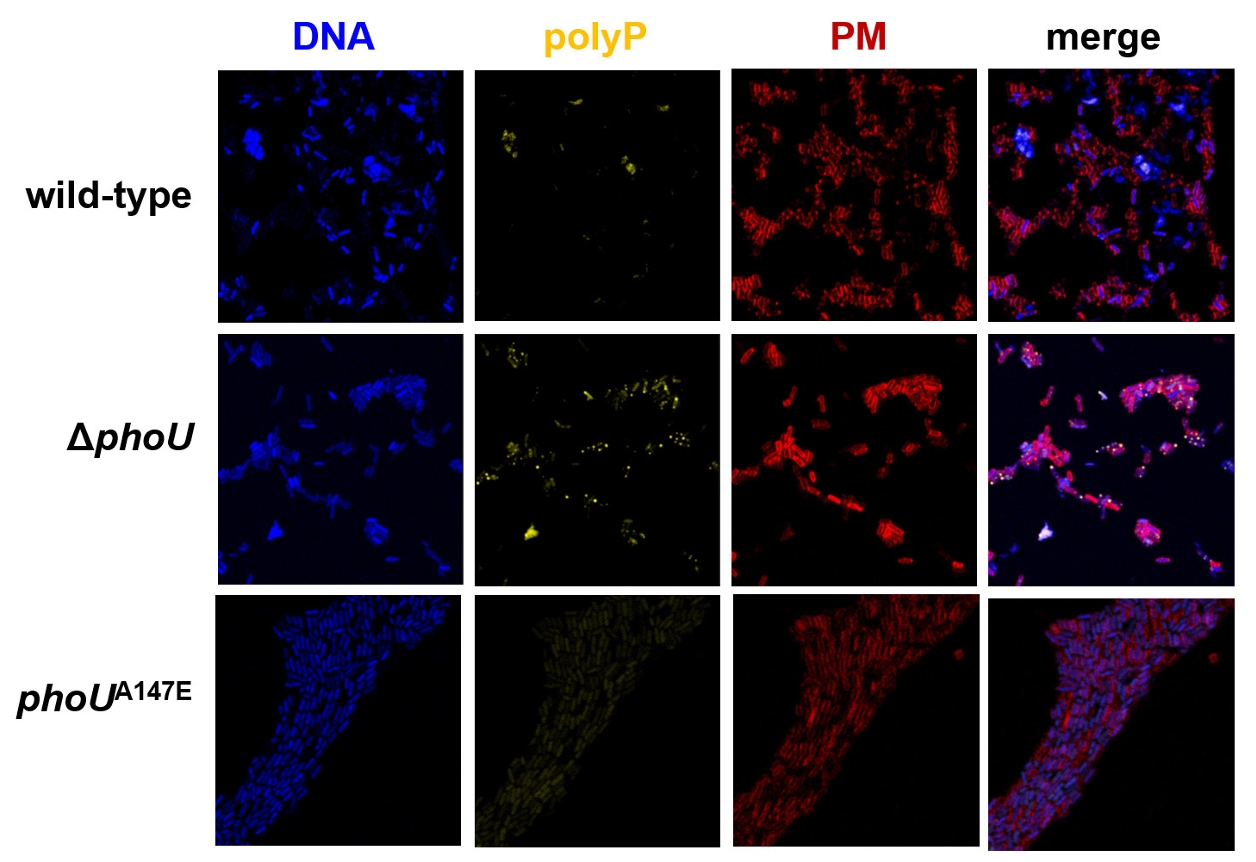
- The pho regulon plays a critical role in maintaining phosphate homeostasis in bacteria, with the PhoU protein functioning as a regulator that bridges the PhoB/PhoR two-component system and the PstSCAB2 phosphate transporter. While PhoU is known to suppress PhoR autophosphorylation under high phosphate conditions via interaction with its PAS domain, its broader regulatory functions remain elusive. Here, we investigated the role of the PhoU Ala147 residue in Salmonella enterica serovar Typhimurium using a phoUA147E substitution mutant. Bacterial two-hybrid and immunoprecipitation assays confirmed that Ala147 is essential for PhoU-PhoR PAS domain interaction, and its substitution leads to derepression of pho regulon genes, even in high phosphate conditions. This disruption impaired Salmonella survival inside macrophages and mouse virulence, demonstrating the importance of PhoU-PhoR interaction in Salmonella pathogenesis. However, unlike the phoU deletion mutant, the phoUA147E mutant does not exhibit growth defects or polyphosphate accumulation, indicating that the PhoU-PhoR interaction is not involved in these phenotypes. Our findings reveal PhoU as a multifaceted regulator, coordinating phosphate uptake and pho regulon expression through distinct molecular interactions, and provide new insights into its role in bacterial physiology and virulence.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) [NRF-2022R1A2B5B02002256, NRF-2022R1A4A1025913, NRF-RS-2025-00561488 to E.-J.L.]; and the Ministry of Education [NRF-RS-2024-00350890, RS-2024-00461215 to S.C.] and a grant from Korea University.
Author Contributions
E.-J.L. designed the research, analyzed the data, and wrote the manuscript; S.B. performed the experiments and wrote the manuscript; S.C. and Y.H. performed experiments; E.C. performed initial experiments; S.P. and J.-S.L. performed mouse experiments.
Conflict of Interest
The authors declare no competing interests.
Ethical Statements
All experimental procedures were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Kangwon National University (approval no. KW-210728-1).
Supplementary Information
Table S1.
Fig. S1.
- Abramson J, Adler J, Dunger J, Evans R, Green T, et al. 2024. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 630: 493–500. ArticlePubMedPMCPDF
- Alix E, Blanc-Potard AB. 2007. MgtC: a key player in intramacrophage survival. Trends Microbiol. 15: 252–256. ArticlePubMed
- Bachhawat P, Swapna GV, Montelione GT, Stock AM. 2005. Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure. 13: 1353–1363. ArticlePubMedPMC
- Baek S, Lee EJ. 2024. PhoU: a multifaceted regulator in microbial signaling and homeostasis. Curr Opin Microbiol. 77: 102401.ArticlePubMed
- Bong D, Sohn J, Lee SJV. 2024. Brief guide to RT-qPCR. Mol Cells. 47: 100141.ArticlePubMedPMC
- Cho BK, Knight EM, Palsson BO. 2006. PCR-based tandem epitope tagging system for Escherichia coli genome engineering. Biotechniques. 40: 67–72. ArticlePubMed
- Choi S, Choi E, Cho YJ, Nam D, Lee J, et al. 2019. The Salmonella virulence protein MgtC promotes phosphate uptake inside macrophages. Nat Commun. 10: 3326.ArticlePubMedPMCPDF
- Choi S, Jeong G, Choi E, Lee EJ. 2022. A dual regulatory role of the PhoU protein in Salmonella Typhimurium. mBio. 13: e0081122. ArticlePubMedPMCLink
- Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 97: 6640–6645. ArticlePubMedPMC
- de Almeida LG, Ortiz JH, Schneider RP, Spira B. 2015. phoU inactivation in Pseudomonas aeruginosa enhances accumulation of ppGpp and polyphosphate. Appl Environ Microbiol. 81: 3006–3015. ArticlePubMedPMCLink
- diCenzo GC, Sharthiya H, Nanda A, Zamani M, Finan TM. 2017. PhoU allows rapid adaptation to high phosphate concentrations by modulating PstSCAB transport rate in Sinorhizobium meliloti. J Bacteriol. 199: e00143–17. ArticlePubMedPMCLink
- Ellison DW, McCleary WR. 2000. The unphosphorylated receiver domain of PhoB silences the activity of its output domain. J Bacteriol. 182: 6592–6597. ArticlePubMedPMCLink
- Gao R, Stock AM. 2015. Temporal hierarchy of gene expression mediated by transcription factor binding affinity and activation dynamics. mBio. 6: e00686–15. ArticlePubMedPMCLink
- García-del Portillo F, Núñez-Hernández C, Eisman B, Ramos-Vivas J. 2008. Growth control in the Salmonella-containing vacuole. Curr Opin Microbiol. 11: 46–52. ArticlePubMed
- Gardner SG, Johns KD, Tanner R, McCleary WR. 2014. The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J Bacteriol. 196: 1741–1752. ArticlePubMedPMCLink
- Gardner SG, Miller JB, Dean T, Robinson T, Erickson M, et al. 2015. Genetic analysis, structural modeling, and direct coupling analysis suggest a mechanism for phosphate signaling in Escherichia coli. BMC Genet. 16(Suppl 2): S2.ArticlePubMedPMCPDF
- Glover RT, Kriakov J, Garforth SJ, Baughn AD, Jacobs WR Jr. 2007. The two-component regulatory system senX3-regX3 regulates phosphate-dependent gene expression in Mycobacterium smegmatis. J Bacteriol. 189: 5495–5503. ArticlePubMedPMCLink
- Hsieh YJ, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol. 13: 198–203. ArticlePubMedPMC
- Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 95: 5752–5756. ArticlePubMedPMC
- Kornberg A, Rao NN, Ault-Riché D. 1999. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 68: 89–125. ArticlePubMed
- Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 32: 461–473. ArticlePubMed
- Lee JW, Lee EJ. 2015. Regulation and function of the Salmonella MgtC virulence protein. J Microbiol. 53: 667–672. ArticlePubMedPDF
- Li Y, Zhang Y. 2007. PhoU is a persistence switch involved in persister formation and tolerance to multiple antibiotics and stresses in Escherichia coli. Antimicrob Agents Chemother. 51: 2092–2099. ArticlePubMedPMCLink
- Lubin EA, Henry JT, Fiebig A, Crosson S, Laub MT. 2016. Identification of the PhoB regulon and role of PhoU in the phosphate starvation response of Caulobacter crescentus. J Bacteriol. 198: 187–200. ArticlePubMedPMCLink
- Makino K, Amemura M, Kawamoto T, Kimura S, Shinagawa H, et al. 1996. DNA binding of PhoB and its interaction with RNA polymerase. J Mol Biol. 259: 15–26. ArticlePubMed
- Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, et al. 1988. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 203: 85–95. ArticlePubMed
- Martín-Martín S, Rodríguez-García A, Santos-Beneit F, Franco-Domínguez E, Sola-Landa A, et al. 2017. Self-control of the PHO regulon: the PhoP-dependent protein PhoU controls negatively expression of genes of PHO regulon in Streptomyces coelicolor. J Antibiot (Tokyo). 71: 113–122. ArticlePubMedPDF
- Morohoshi T, Maruo T, Shirai Y, Kato J, Ikeda T, et al. 2002. Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl Environ Microbiol. 68: 4107–4110. ArticlePubMedPMCLink
- Muda M, Rao N, Torriani A. 1992. Role of PhoU in phosphate transport and alkaline phosphatase regulation. J Bacteriol. 174: 8057–8064. ArticlePubMedPMCLink
- Piepenbreier H, Fritz G, Gebhard S. 2017. Transporters as information processors in bacterial signalling pathways. Mol Microbiol. 104: 1–15. ArticlePubMedLink
- Rao NN, Gomez-Garcia MR, Kornberg A. 2009. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 78: 605–647. ArticlePubMed
- Röder J, Felgner P, Hensel M. 2021. Single-cell analyses reveal phosphate availability as critical factor for nutrition of Salmonella enterica within mammalian host cells. Cell Microbiol. 23: e13374. ArticlePubMed
- Schramke H, Laermann V, Tegetmeyer HE, Brachmann A, Jung K, et al. 2017. Revisiting regulation of potassium homeostasis in Escherichia coli: the connection to phosphate limitation. Microbiologyopen. 6: e00438. ArticlePubMedPMCLink
- Steed PM, Wanner BL. 1993. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol. 175: 6797–6809. ArticlePubMedPMCLink
- Tang J, Chen J, Liu Y, Hu J, Xia Z, et al. 2022. The global regulator PhoU positively controls growth and butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. Front Microbiol. 13: 904627.ArticlePubMedPMC
- Torriani A, Rothman F. 1961. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 81: 835–836. ArticlePubMedPMCLink
- Wang X, Han H, Lv Z, Lin Z, Shang Y, et al. 2017. PhoU2 but not PhoU1 as an important regulator of biofilm formation and tolerance to multiple stresses by participating in various fundamental metabolic processes in Staphylococcus epidermidis. J Bacteriol. 199: e00219-17.ArticlePubMedPMCLink
- Wang C, Mao Y, Yu J, Zhu L, Li M, et al. 2013. PhoY2 of mycobacteria is required for metabolic homeostasis and stress response. J Bacteriol. 195: 243–252. ArticlePubMedPMCLink
References
Figure & Data
References
Citations

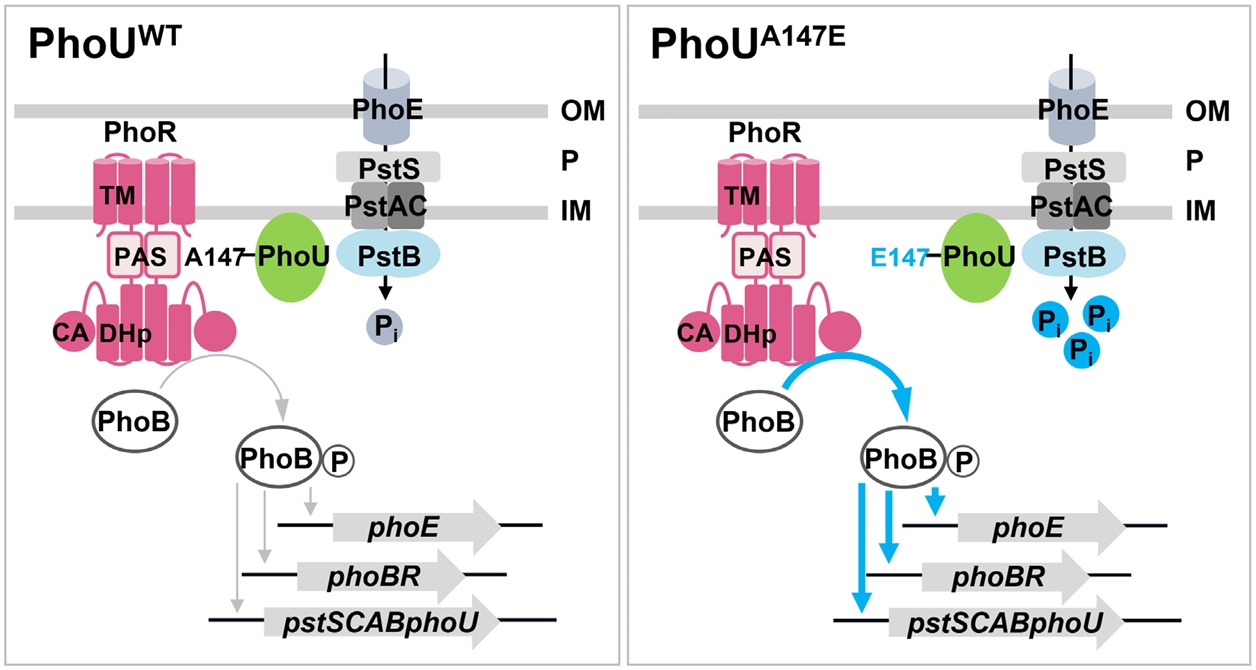
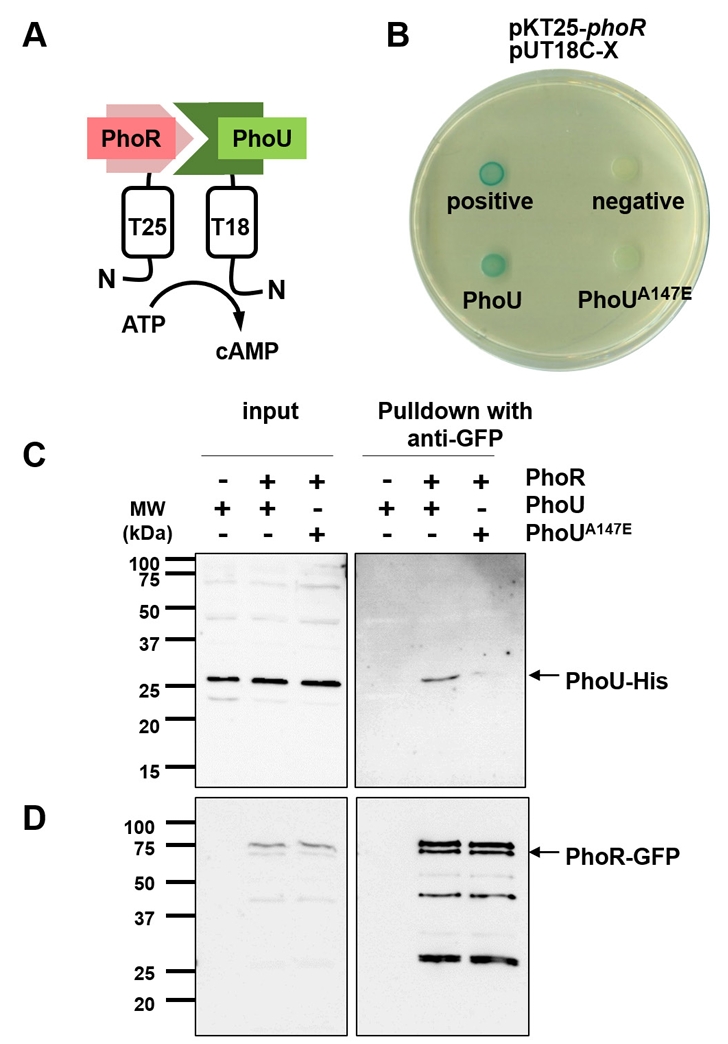
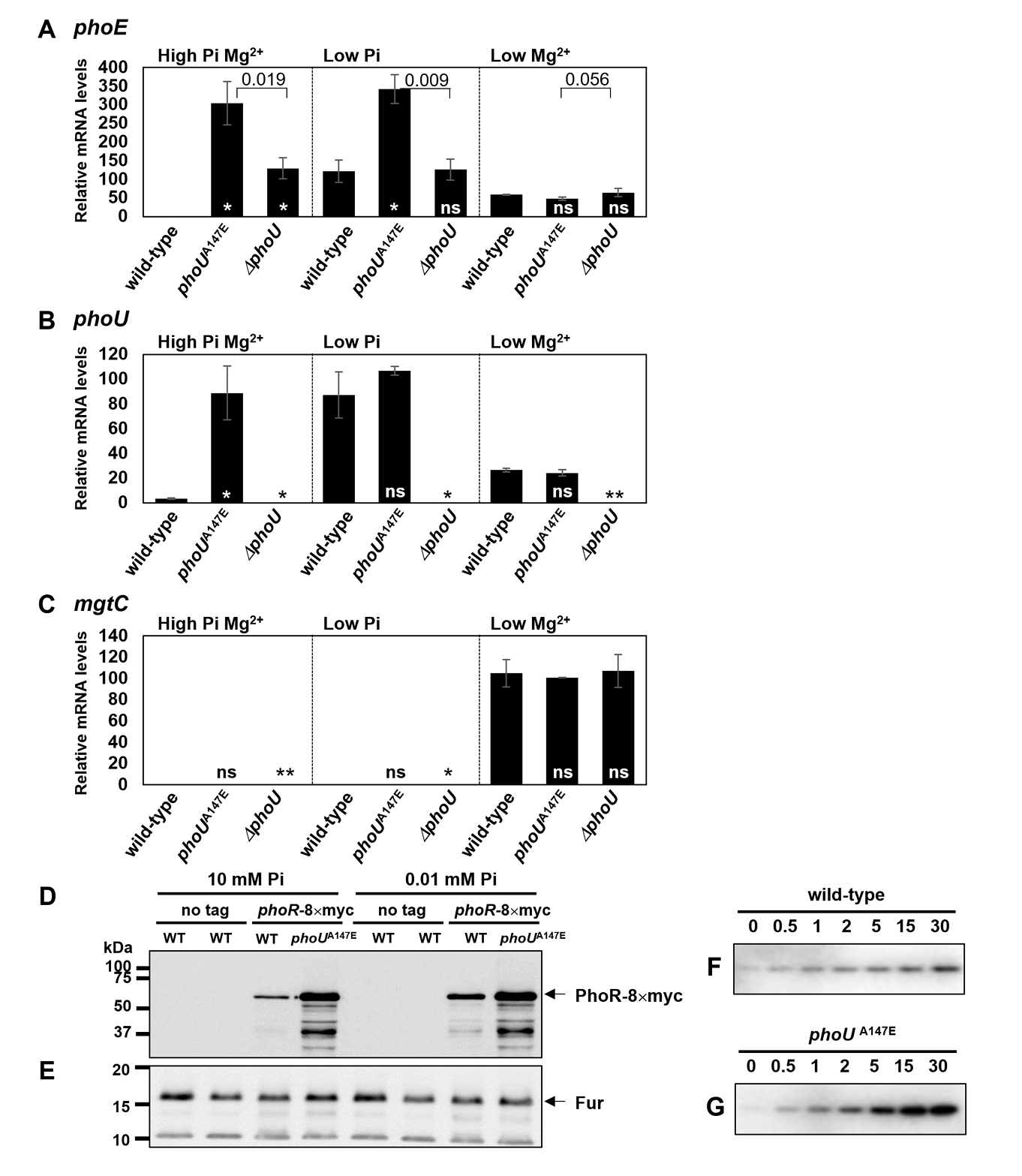
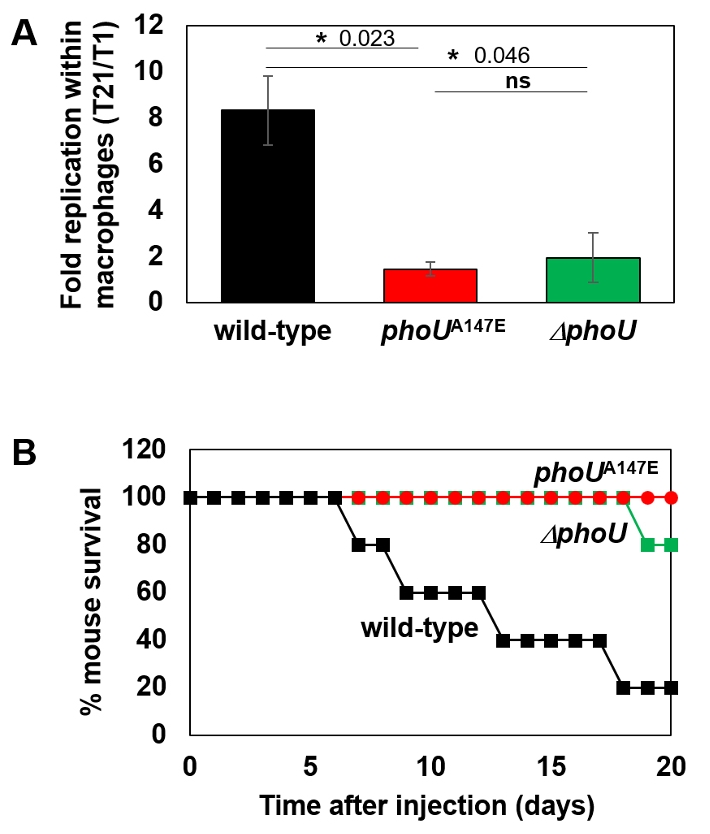
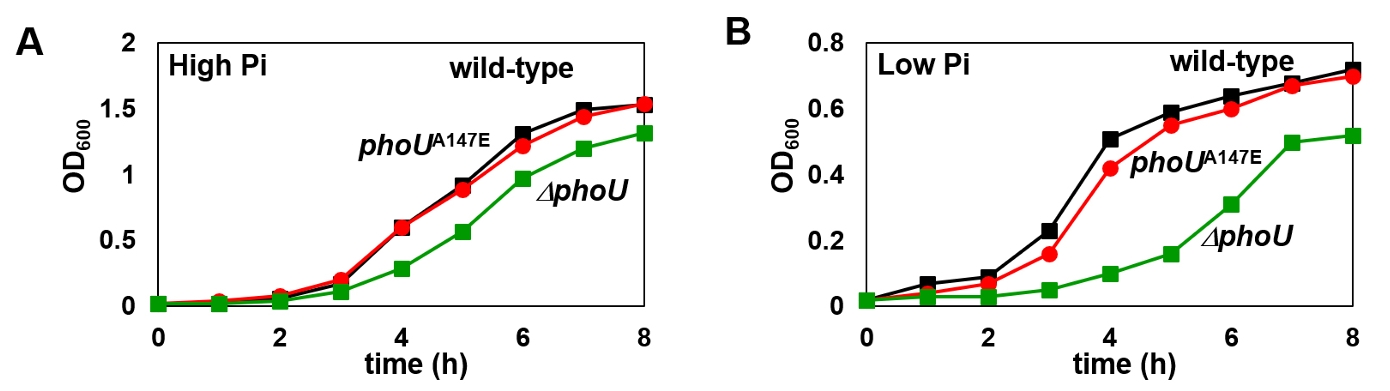
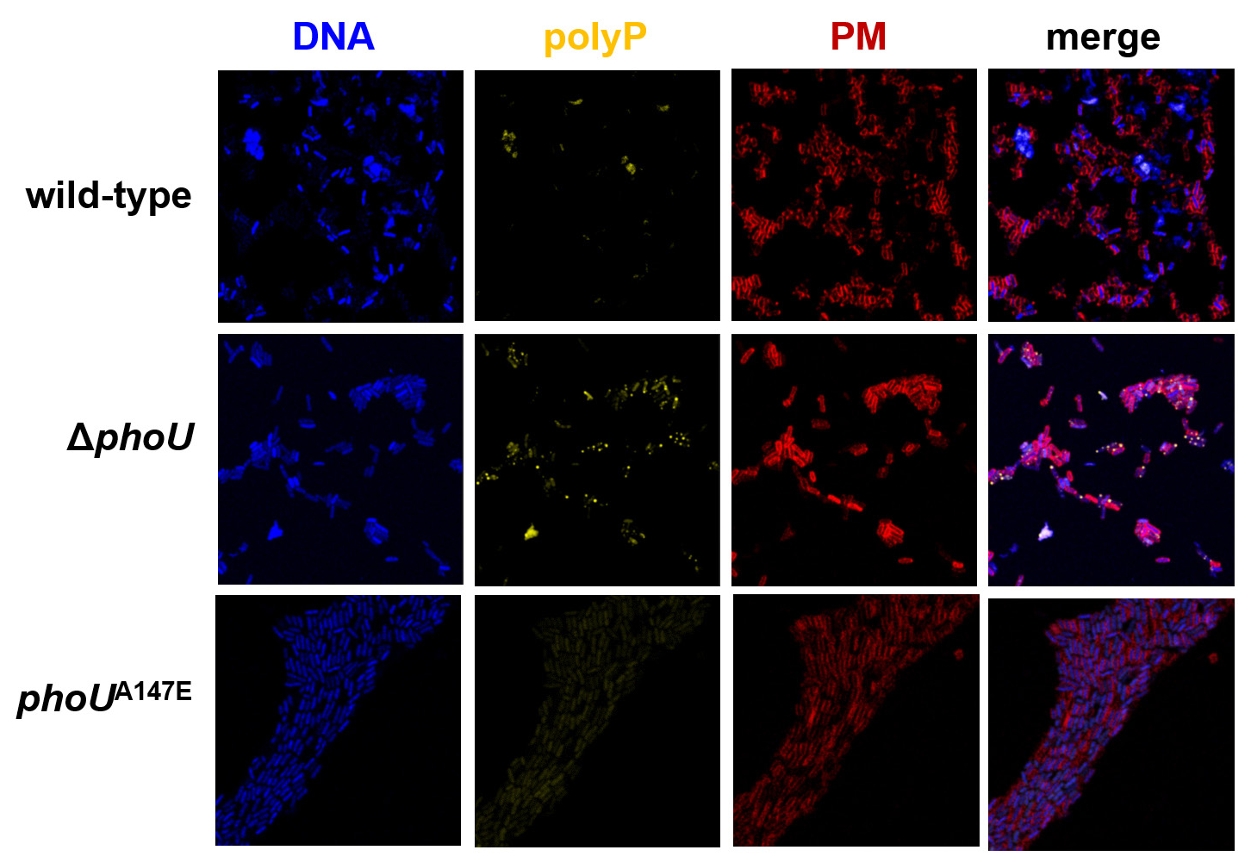
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article







