Articles
- Page Path
- HOME > J. Microbiol > Volume 63(10); 2025 > Article
-
Full article
Mannose phosphotransferase system subunit IID of Streptococcus mutans elicits maturation and activation of dendritic cells - Sungho Jeong1, Chaeyeon Park1, Dongwook Lee1, Hyun Jung Ji2, Ho Seong Seo2, Cheol-Heui Yun3,4, Jintaek Im1,*, Seung Hyun Han1,*
-
Journal of Microbiology 2025;63(10):e2505014.
DOI: https://doi.org/10.71150/jm.2505014
Published online: October 31, 2025
1Department of Oral Microbiology and Immunology, and Dental Research Institute, School of Dentistry, Seoul National University, Seoul 08826, Republic of Korea
2Research Division for Biotechnology, Korea Atomic Energy Research Institute, Jeongeup 56212, Republic of Korea
3Department of Agricultural Biotechnology, and Research Institute of Agriculture and Life Sciences, Seoul National University, Seoul 08826, Republic of Korea
4Institutes of Green Bio Science and Technology, Seoul National University, Pyeongchang 25354, Republic of Korea
- *For correspondence. Jintaek Im jintaek1@snu.ac.kr Seung Hyun Han shhan-mi@snu.ac.kr
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,093 Views
- 33 Download
ABSTRACT
- Streptococcus mutans is a Gram-positive pathogen that causes dental caries and subsequent pulpal infection leading to pulpitis. Although dendritic cells (DCs) are known to be involved in disease progression and immune responses during S. mutans infection, little is known about which component of S. mutans is responsible for the DC responses. Although the mannose phosphotransferase system (Man-PTS) is the primary sugar transporter of S. mutans, it is also a potential virulence factor. Since Man-PTS subunit IID (ManIID) embedded on the bacterial membrane is indispensable for Man-PTS function, we investigated its role in the maturation and activation of DCs stimulated with a ManIID-deficient strain (Δpts) of S. mutans and recombinant ManIID (rManIID) protein. When mouse bone marrow-derived DCs were treated with heat-killed S. mutans wild-type (WT) or Δpts, bacterial adherence and internalization of Δpts were lower than those of WT. Moreover, the heat-killed S. mutans Δpts strain was inferior to the wild-type in inducing expression of phenotypic maturation markers, such as CD80, CD86, MHC-I, and MHC-II, and proinflammatory cytokine, IL-6. In line with the trends in marker expression, the endocytic capacity of DCs treated with the Δpts strain was comparable to that of untreated DCs whereas DCs treated with the WT strain dose-dependently lost their endocytic capacity. Furthermore, rManIID dose-dependently promoted both phenotypic maturation marker expression and IL-6 production by DCs. Collectively, these results demonstrate that ManIID plays a crucial role in the adhesion and internalization of S. mutans into DCs and is one of the major immune-stimulating agents responsible for maturation and activation of DCs during S. mutans infection.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2022M3A9F3082330, NRF-2023R1A2C1004987, RS-2022-00164722, and RS-2025-00518511).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Statements
The animal study was approved by the Seoul National University Institutional Animal Care and Use Committee (Approval Number: SNU-210403-1-2) and experiments were performed according to the guidelines provided by the institution.
- Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol. 188: 3748–3756. ArticlePubMedPMCPDF
- Ajdić D, Pham T. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J Bacteriol. 189: 5049–5059. ArticlePubMedPMCPDF
- Akamp T, Rosendahl A, Galler M, Wölflick M, Buchalla W, et al. 2024. An in vitro coculture approach to study the interplay between dental pulp cells and Streptococcus mutans. Int Endod J. 57: 164–177. ArticlePubMed
- Alves LA, Nomura R, Mariano FS, Harth-Chu EN, Stipp RN, et al. 2016. CovR regulates Streptococcus mutans susceptibility to complement immunity and survival in blood. Infect Immun. 84: 3206–3219. ArticlePubMedPMCPDF
- Butcher JP, Malcolm J, Benson RA, Deng DM, Brewer JM, et al. 2011. Effects of Streptococcus mutans on dendritic cell activation and function. J Dent Res. 90: 1221–1227. ArticlePubMedPDF
- Dudek AM, Martin S, Garg AD, Agostinis P. 2013. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol. 4: 438.ArticlePubMedPMC
- Embgenbroich M, van der Zande HJP, Hussaarts L, Schulte-Schrepping J, Pelgrom R, et al. 2021. Soluble mannose receptor induces proinflammatory macrophage activation and metaflammation. Proc Natl Acad Sci USA. 118: e2103304118. ArticlePubMedPMC
- Forssten SD, Björklund M, Ouwehand AC. 2010. Streptococcus mutans, caries and simulation models. Nutrients. 2: 290–298. ArticlePubMedPMC
- Hahn CL, Liewehr FR. 2007. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod. 33: 213–219. ArticlePubMed
- Hahn CL, Schenkein HA, Tew JG. 2005. Endocarditis-associated oral streptococci promote rapid differentiation of monocytes into mature dendritic cells. Infect Immun. 73: 5015–5021. ArticlePubMedPMCPDF
- Hament JM, Aerts PC, Fleer A, Van Dijk H, Harmsen T, et al. 2004. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory syncytial virus. Pediatr Res. 55: 972–978. ArticlePubMed
- Han HS, Shin HR, Kim S, Cho YD. 2025. Polynucleotide with cross-linked hyaluronic acid reduces inflammation and increases collagen synthesis. J Periodontal Implant Sci. 55: 206–216. ArticlePubMedPMCPDF
- Harmon MA, Tew JG, Best AM, Hahn CL. 2009. Mature dendritic cells in inflamed human pulps beneath deep caries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 107: 727–732. ArticlePubMed
- Horst OV, Horst JA, Samudrala R, Dale BA. 2011. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 12: 9.ArticlePubMedPMCPDF
- Ikeda T, Sandham HJ. 1971. Prevalence of Streptococcus mutans on various tooth surfaces in negro children. Arch Oral Biol. 16: 1237–1240. ArticlePubMed
- Jeckelmann JM, Erni B. 2020. The mannose phosphotransferase system (Man-PTS) - mannose transporter and receptor for bacteriocins and bacteriophages. Biochim Biophys Acta Biomembr. 1862: 183412.ArticlePubMed
- Kim SK, Im J, Ko EB, Lee D, Seo HS, et al. 2023. Lipoteichoic acid of Streptococcus gordonii as a negative regulator of human dendritic cell activation. Front Immunol. 14: 1056949.ArticlePubMedPMC
- Kim SK, Im J, Yun CH, Son JY, Son CG, et al. 2008. Armillariella mellea induces maturation of human dendritic cells without induction of cytokine expression. J Ethnopharmacol. 119: 153–159. ArticlePubMed
- Kim HY, Kim SK, Seo HS, Jeong S, Ahn KB, et al. 2018. Th17 activation by dendritic cells stimulated with gamma-irradiated Streptococcus pneumoniae. Mol Immunol. 101: 344–352. ArticlePubMed
- Kim HJ, Yang JS, Woo SS, Kim SK, Yun CH, et al. 2007. Lipoteichoic acid and muramyl dipeptide synergistically induce maturation of human dendritic cells and concurrent expression of proinflammatory cytokines. J Leukoc Biol. 81: 983–989. ArticlePubMedPDF
- Ko EB, Kim SK, Seo HS, Yun CH, Han SH. 2017. Serine-rich repeat adhesins contribute to Streptococcus gordonii-induced maturation of human dendritic cells. Front Microbiol. 8: 523.ArticlePubMedPMC
- Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, et al. 2012. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep. 2: 332.ArticlePubMedPMCPDF
- Kwabena Danso I, Woo JH, Baek SH, Kim K, Lee K. 2024. Pulmonary toxicity assessment of polypropylene, polystyrene, and polyethylene microplastic fragments in mice. Toxicol Res. 40: 313–323. ArticlePubMedPMCPDF
- Lapteva N, Seethammagari MR, Hanks BA, Jiang J, Levitt JM, et al. 2007. Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 67: 10528–10537. ArticlePubMedPDF
- Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, et al. 2019. The biology of Streptococcus mutans. Microbiol Spectr. 7: gpp3-0051-2018.ArticlePDF
- Li H, Wang D. 2014. Streptococcus mutans wall-associated protein a promotes TLR4-induced dendritic cell maturation. Scand J Immunol. 80: 121–126. ArticlePubMed
- Liang X, Ji Y. 2006. Alpha-toxin interferes with integrin-mediated adhesion and internalization of Staphylococcus aureus by epithelial cells. Cell Microbiol. 8: 1656–1668. ArticlePubMed
- Lim S, Seo HS, Jeong J, Yoon H. 2019. Understanding the multifaceted roles of the phosphoenolpyruvate: Phosphotransferase system in regulation of Salmonella virulence using a mutant defective in ptsI and crr expression. Microbiol Res. 223-225: 63–71. ArticlePubMed
- Lu X, Liu T, Zhou J, Liu J, Yuan Z, et al. 2022. Subgingival microbiome in periodontitis and type 2 diabetes mellitus: An exploratory study using metagenomic sequencing. J Periodontal Implant Sci. 52: 282–297. ArticlePubMedPMCPDF
- Luqman A, Ohlsen K. 2025. Cytokine-mediated inhibition of Staphylococcus aureus adherence and invasion into nonphagocytic cells. Med Microbiol Immunol. 214: 31.ArticlePubMedPMCPDF
- Maisonneuve E, Chevrier J, Dubus M, Varin J, Sergheraert J, et al. 2020. Infection of human dental pulp stromal cells by Streptococcus mutans: Shedding light on bacteria pathogenicity and pulp inflammation. Front Cell Dev Biol. 8: 785.ArticlePubMedPMC
- Marple AC, Shannon BA, Rishi A, Estafanos L, Armstrong BD, et al. 2025. The Streptococcus pyogenes mannose phosphotransferase system (Man-PTS) influences antimicrobial activity and niche-specific nasopharyngeal infection. J Bacteriol. 207: e0049224. ArticlePubMedPDF
- Matsumoto-Nakano M. 2018. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev. 54: 22–29. ArticlePubMed
- Moye ZD, Burne RA, Zeng L. 2014. Uptake and metabolism of N-acetylglucosamine and glucosamine by Streptococcus mutans. Appl Environ Microbiol. 80: 5053–5067. ArticlePubMedPMCPDF
- Nobbs A. 2017. Getting to the heart of the matter: Role of Streptococcus mutans adhesin Cnm in systemic disease. Virulence. 8: 1–4. ArticlePubMed
- Oiki S, Nakamichi Y, Maruyama Y, Mikami B, Murata K, et al. 2019. Streptococcal phosphotransferase system imports unsaturated hyaluronan disaccharide derived from host extracellular matrices. PLoS One. 14: e0224753. ArticlePubMedPMC
- Panjaitan D, Horng T, Chien CC, Yang HC, You RI, et al. 2021. The PTS components in Klebsiella pneumoniae affect bacterial capsular polysaccharide production and macrophage phagocytosis resistance. Microorganisms. 9: 335.ArticlePubMedPMC
- Popović N, Djokić J, Brdarić E, Dinić M, Terzić-Vidojević A, et al. 2019. The influence of heat-killed Enterococcus faecium BGPAS1-3 on the tight junction protein expression and immune function in differentiated Caco-2 cells infected with Listeria monocytogenes ATCC 19111. Front Microbiol. 10: 412.ArticlePubMedPMC
- Quispe-Salcedo A, Ohshima H. 2021. The role of dendritic cells during physiological and pathological dentinogenesis. J Clin Med. 10: 3348.ArticlePubMedPMC
- Reis e Sousa C, Sher A, Kaye P. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 11: 392–399. ArticlePubMed
- Sato Y, Okamoto-Shibayama K, Azuma T. 2015. Additional glucose-PTS induction in Streptococcus mutans mutant deficient in mannose- and cellobiose-PTS. Bull Tokyo Dent Coll. 56: 185–188. ArticlePubMed
- Vadeboncoeur C, Pelletier M. 1997. The phosphoenolpyruvate: sugar phosphotransferase system of oral Streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 19: 187–207. ArticlePubMed
- van der Zande HJP, Nitsche D, Schlautmann L, Guigas B, Burgdorf S. 2021. The mannose receptor: From endocytic receptor and biomarker to regulator of (meta)inflammation. Front Immunol. 12: 765034.ArticlePubMedPMC
- Wollenberg A, Mommaas M, Oppel T, Schottdorf M, Gunther S, et al. 2002. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 118: 327–334. ArticlePubMed
- Xiao Q, Xia Y. 2023. Insights into dendritic cell maturation during infection with application of advanced imaging techniques. Front Cell Infect Microbiol. 13: 1140765.ArticlePubMedPMC
- Xu Q, Katz J, Zhang P, Ashtekar R, Gaddis E, et al. 2011. Contribution of a Streptococcus mutans antigen expressed by a Salmonella vector vaccine in dendritic cell activation. Infect Immun. 79: 3792–3800. ArticlePubMedPMCPDF
- Yang J, Woo SS, Ryu YH, Yun CH, Cho MH, et al. 2009. Bacillus anthracis lethal toxin attenuates lipoteichoic acid-induced maturation and activation of dendritic cells through a unique mechanism. Mol Immunol. 46: 3261–3268. ArticlePubMed
- Yin X, Chen S, Eisenbarth SC. 2021. Dendritic cell regulation of T helper cells. Annu Rev Immunol. 39: 759–790. ArticlePubMed
- Zeng L, Chakraborty B, Farivar T, Burne A. 2017. Coordinated regulation of the EIIman and fruRKI operons of Streptococcus mutans by global and fructose-specific pathways. Appl Environ Microbiol. 83: e01403–17. ArticlePubMedPMCPDF
- Zhang R, Becnel L, Li M, Chen C, Yao Q. 2006. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur J Immunol. 36: 2993–3006. ArticlePubMed
- Zhou W, Wang G, Wang C, Ren F, Hao Y. 2016. Both IIC and IID components of mannose phosphotransferase system are involved in the specific recognition between immunity protein PedB and bacteriocin-receptor complex. PLoS One. 11: e0164973. ArticlePubMedPMC
References
Figure & Data
References
Citations

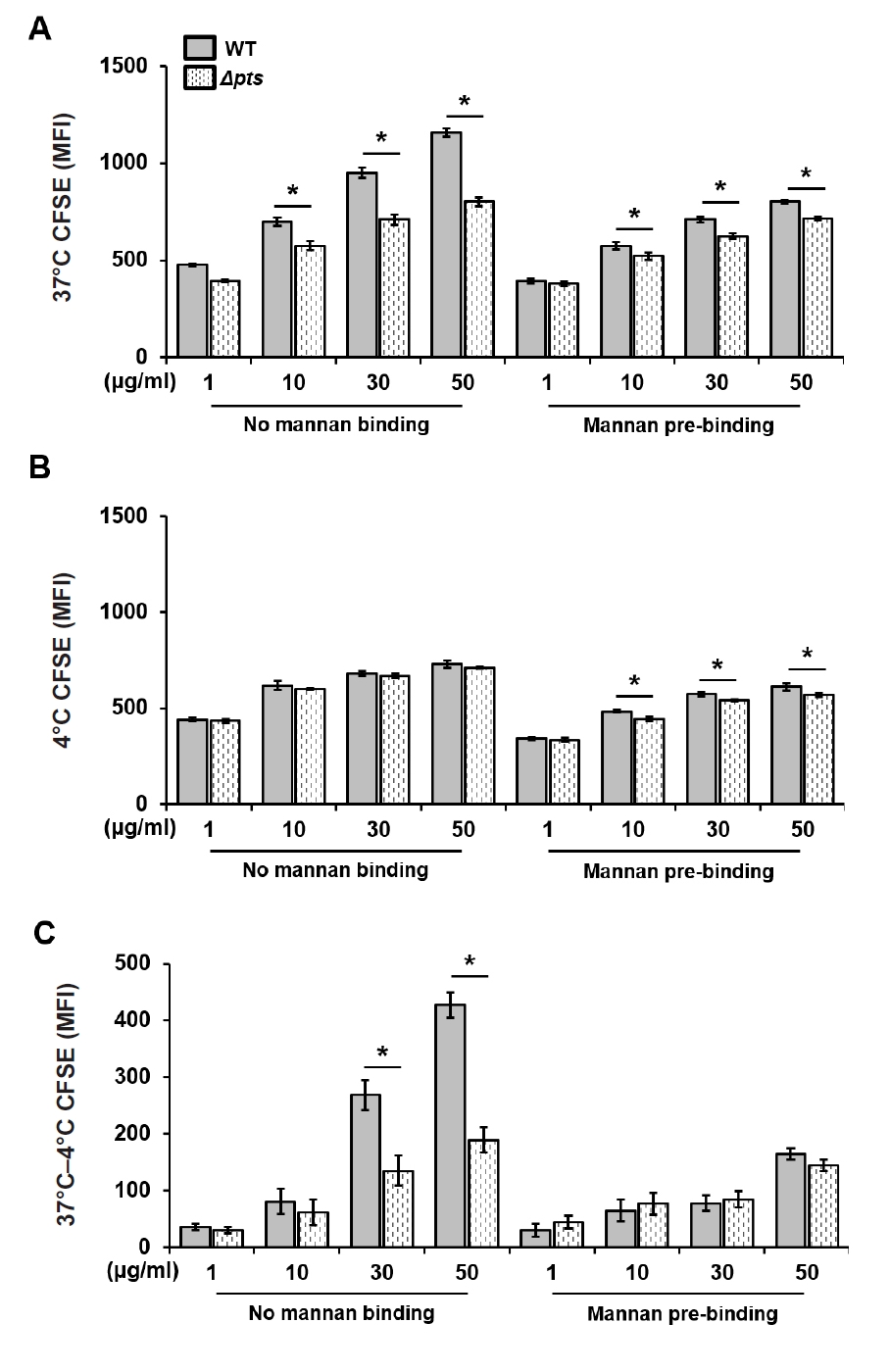
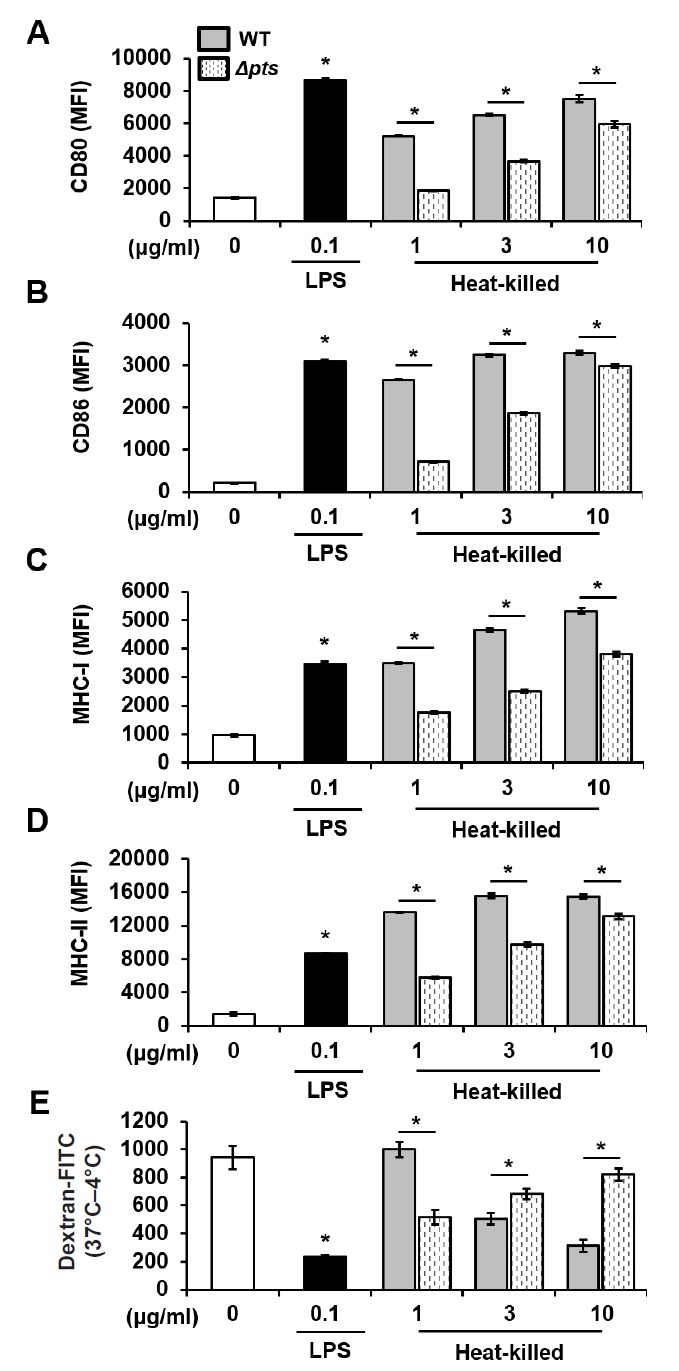
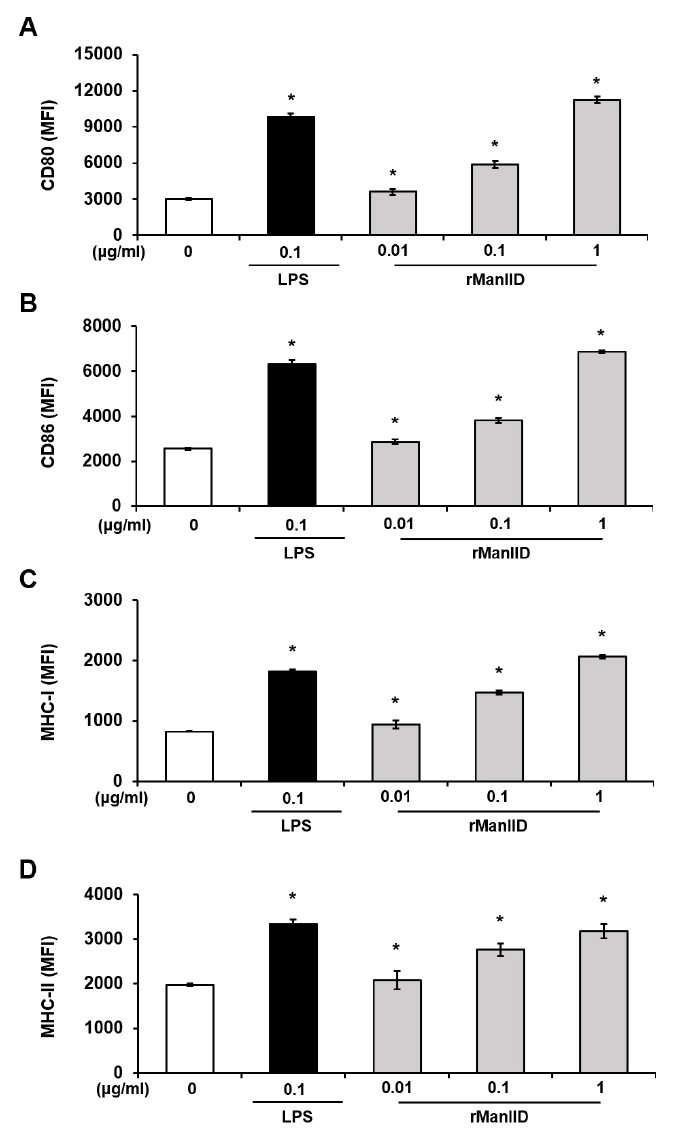
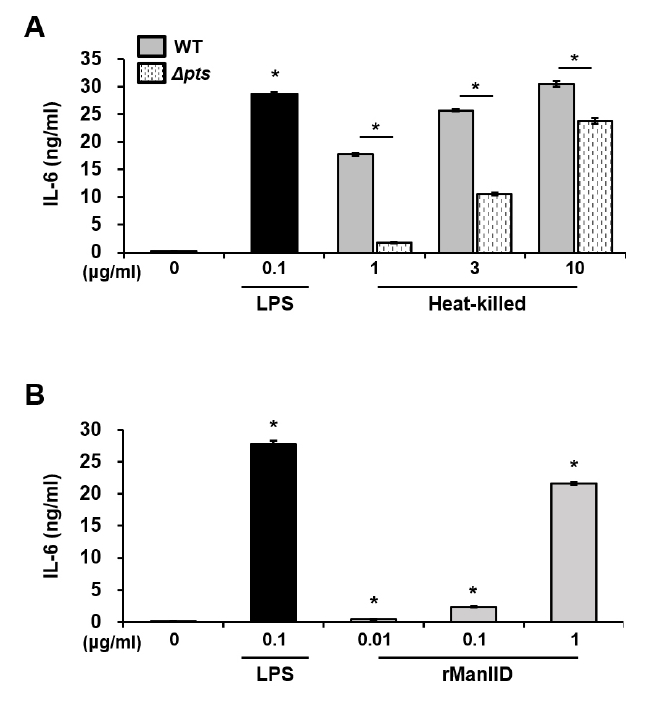
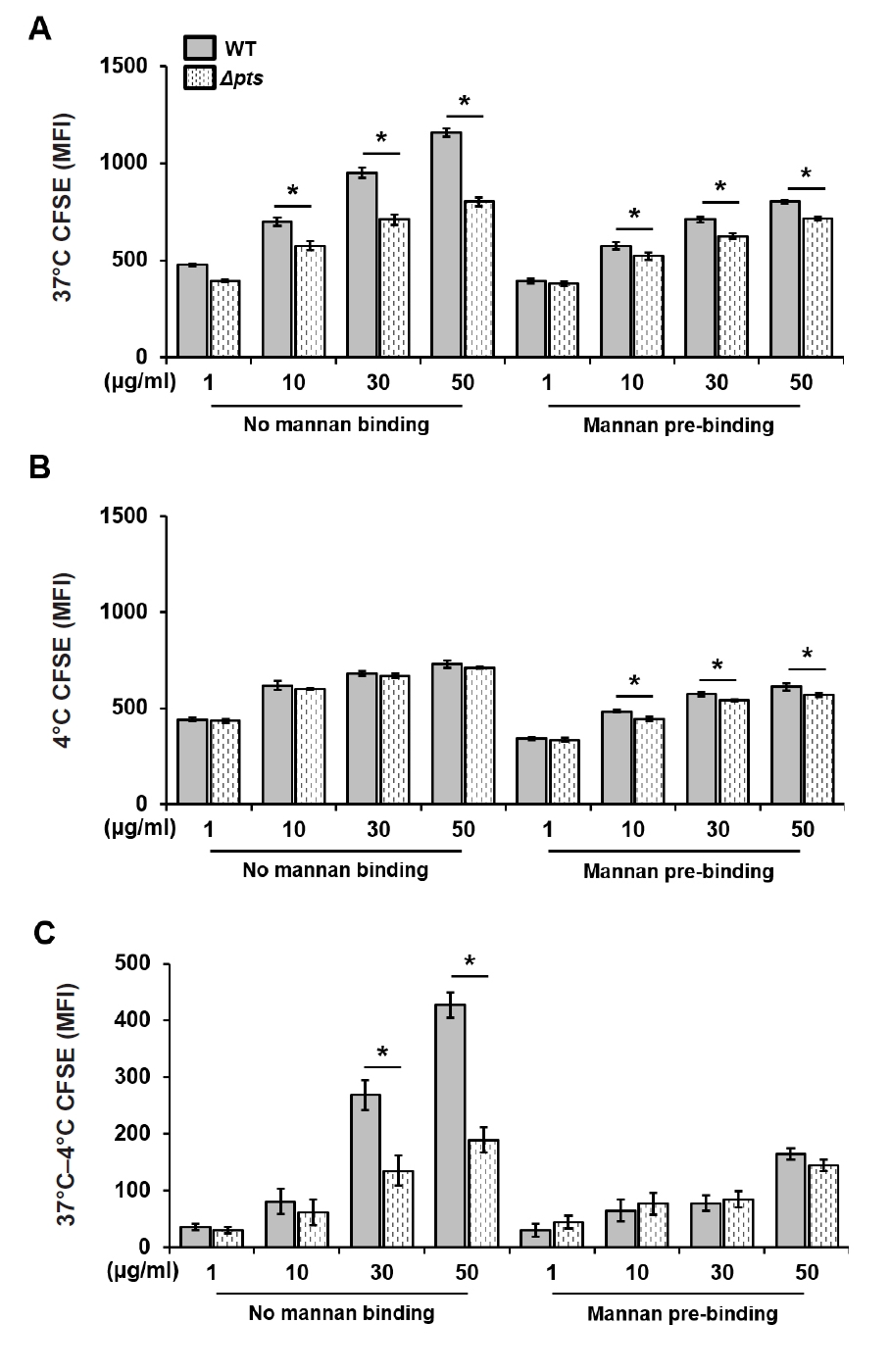
Fig. 1.
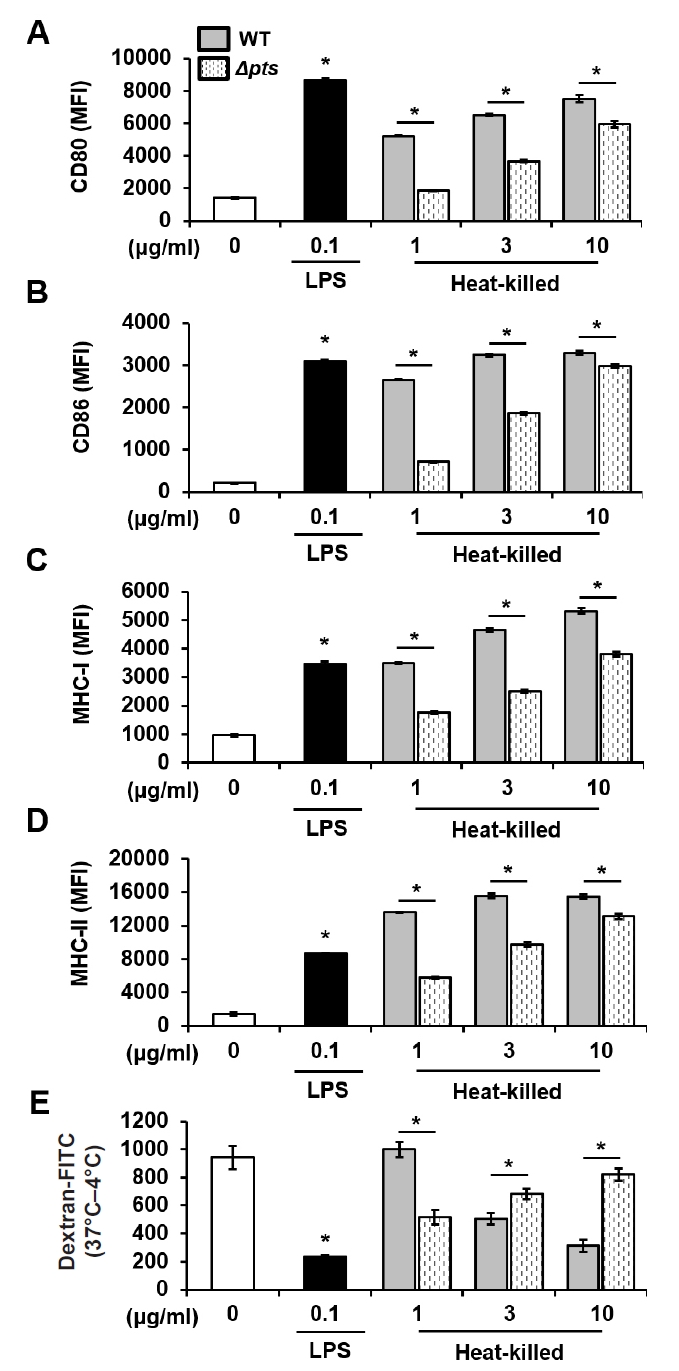
Fig. 2.
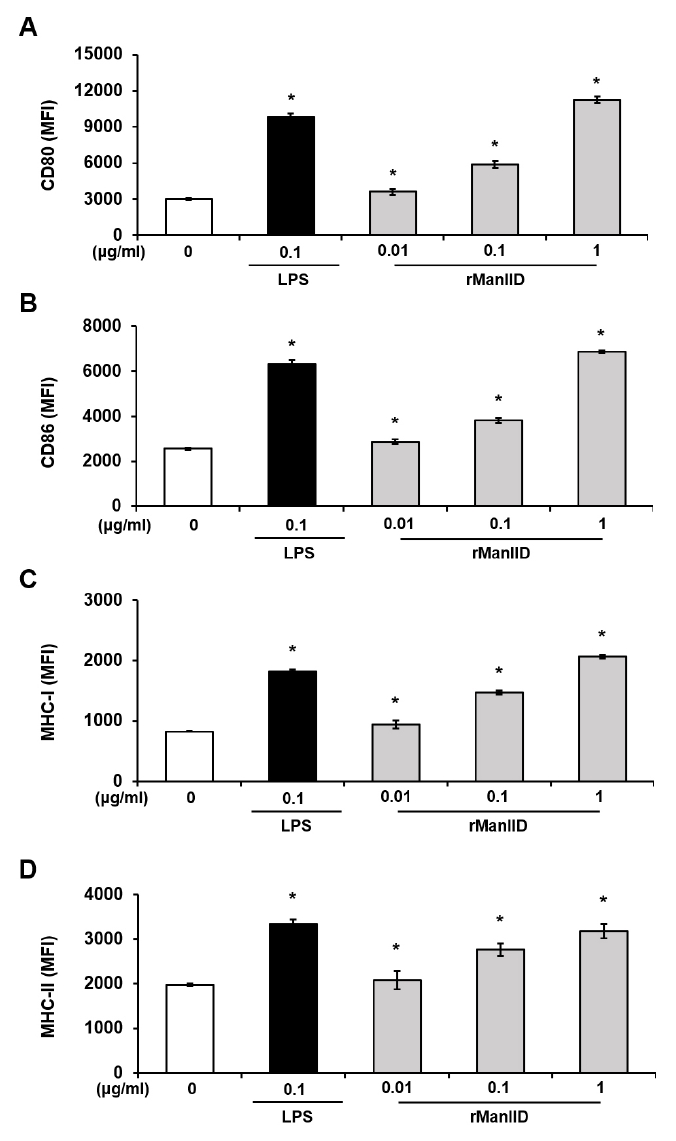
Fig. 3.
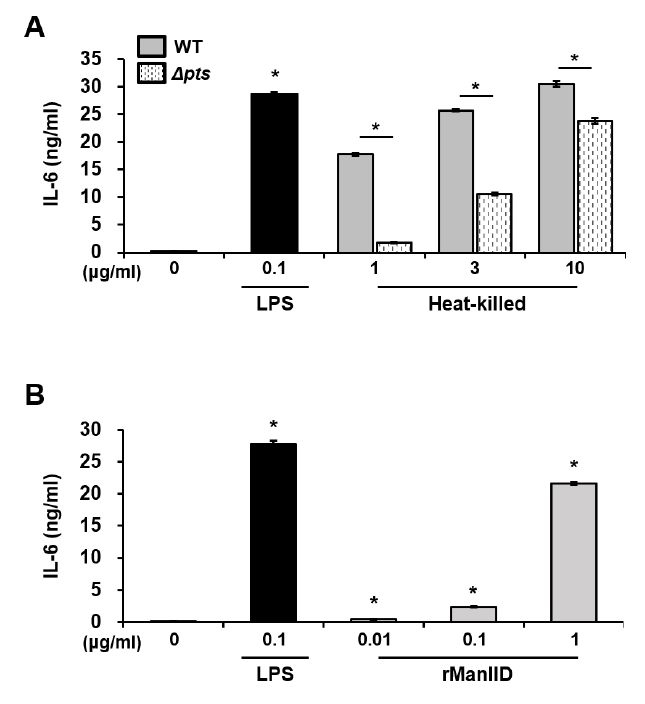
Fig. 4.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article





