ABSTRACT
- Two Gram-stain-negative, strictly aerobic, non-motile, rod-shaped bacteria, designated D3-12ᵀ and G2-2ᵀ, were isolated from the phycosphere of marine red algae. Both strains exhibited catalase- and oxidase-positive activities. Strain D3-12ᵀ grew optimally at 30°C, pH 7.0, and 2.0–3.0% (w/v) NaCl, while strain G2-2ᵀ showed optimal growth at 30°C, pH 7.0, and 2.0% NaCl. Ubiquinone-10 was the sole respiratory quinone in both strains. The major fatty acids (> 5%) in strain D3-12ᵀ were feature 8 (C18:1 ω7c and/or C18:1 ω6c), 11-methyl-C18:1 ω7c, and C16:0, while strain G2-2ᵀ contained summed feature 8 and C16:0. The predominant polar lipids in strain D3-12ᵀ were phosphatidylcholine, phosphatidylglycerol, and phosphatidylethanolamine, whereas strain G2-2ᵀ contained phosphatidylglycerol and diphosphatidylglycerol. The genomic DNA G + C content was 59.9% for strain D3-12ᵀ and 60.2% for strain G2-2ᵀ. Phylogenetic analyses based on 16S rRNA and whole-genome sequences placed both strains into distinct lineages within the family Roseobacteraceae, separate from previously described genera. Genome-based relatedness metrics, including average nucleotide identity, digital DNA-DNA hybridization, average amino acid identity, and percentage of conserved proteins, further confirmed that these strains represent novel genera. Based on phenotypic, chemotaxonomic, and molecular characteristics, strains D3-12ᵀ and G2-2ᵀ are proposed as novel genera: Phycobium rhodophyticola gen. nov., sp. nov. (D3-12ᵀ = KACC 22712ᵀ = JCM 35528ᵀ) and Aliiphycobium algicola gen. nov., sp. nov. (G2-2ᵀ = KACC 22602ᵀ = JCM 35752ᵀ). Additionally, metabolic features relevant to interactions with marine algae, including genes associated with carbohydrate-active enzymes, vitamin biosynthesis, phenylacetic acid production, and bacterioferritin synthesis, were bioinformatically investigated.
-
Keywords: Phycobium rhodophyticola, Aliiphycobium algicola, marine red algae, new taxa (Pseudomonadota), metabolic interaction, phycosphere
Introduction
The family Roseobacteraceae within the phylum Pseudomonadota was recently established based on whole-genome phylogenetic and genotypic analyses of roseobacter clade members, which were originally classified as a broad group of marine bacteria within the family Rhodobacteraceae (Liang et al., 2021). As of May 2025, Roseobacteraceae comprises 137 validly published genera (https://lpsn.dsmz.de/family/roseobacteraceae) exhibiting diverse phenotypic, physiological, and ecological traits, which may contribute to marine biogeochemical cycles, including sulfur and carbon cycling, aerobic anoxygenic photosynthesis, and carbon monoxide oxidation, and symbiotic interactions with both micro- and macro-organisms in marine environments (Ding et al., 2023; Geng and Belas, 2010). Roseobacteraceae strains have been isolated from various marine environments, including seawater (Hwang and Cho, 2008), tidal mudflats (Lee et al., 2020), sea sand (Thongphrom et al., 2017), marine solar saltern (Wang et al., 2023), biofilms (Cui et al., 2025), and polar sea ice (Gosink et al., 1997). Notably, many Roseobacteraceae members have been associated with marine algae, including dinoflagellates (Yang et al., 2018), diatoms (Crenn et al., 2016), microalgae (Jung et al., 2019), and other macroalgae (Han et al., 2024; Lee et al., 2024a).
The marine coastal regions are known for their high biodiversity, including fish, macroalgae, diatoms, and microorganisms (Tak et al., 2024; Yang et al., 2024). In particular, macroalgae serve as crucial habitats for various marine organisms, including fish and microorganisms (Evans et al., 2014; Lee et al., 2024b), and are also commercially important as food sources (Wells et al., 2017). Bacteria in the phycosphere play essential roles in algal growth and development. Marine red algae (phylum Rhodophyta) represent one of the oldest and most diverse groups of marine algae, comprising over 7,000 recognized species and contributing significantly to primary production (Guiry, 2024), leading to increasing research on metabolic interactions between bacteria and marine red algae (Cirri and Pohnert, 2019; Kim et al., 2024b). As part of our ongoing research on these interactions, we have isolated numerous novel bacterial strains from the phycosphere of marine red macroalgae (Bayburt et al., 2024; Jin et al., 2023; Kim et al., 2024a; Lee et al., 2024c). In this study, we also isolated two putative novel genus strains within the family Roseobacteraceae from marine red macroalgae and characterized their taxonomy using a polyphasic approach.
Materials and Methods
Isolation of bacterial strains
Strains D3-12ᵀ and G2-2ᵀ were isolated from the phycosphere of the marine red algae Melanothamnus japonicus and Chondrus sp., respectively, collected from the coastal regions of Daejin (38°30′14″N, 128°25′36″E) and Gonghyeonjin (38°21′21″N, 128°30′45″E) in Gangwon Province, Republic of Korea, as previously described (Kim et al., 2024a). Briefly, the collected algal samples were thoroughly washed with artificial seawater (ASW; 20.0 g NaCl, 2.9 g MgSO4, 4.5 g MgCl2·6H2O, 0.6 g KCl, and 1.8 g CaCl2·2H2O per liter) using mechanical vortexing to remove loosely attached microorganisms. The algae were then homogenized using a mechanical homogenizer and serially diluted in ASW. Aliquots of 100 μl from each dilution were spread onto marine agar (MA; MBcell, Korea) and incubated aerobically at 25°C for 7 days. Colonies grown on MA were screened by PCR amplification of the 16S rRNA gene using universal primers 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) (Lane, 1991). PCR products were digested with the restriction enzymes HaeIII and HhaI, and the resulting fragment patterns were analyzed on a 2% agarose gel. Representative amplicons showing unique or identical fragment patterns were partially sequenced using the universal primer 340F (5′-CCT ACG GGA GGC AGC AG-3′). The obtained sequences were compared with those of validly published type strains using the EzBioCloud server (https://www.ezbiocloud.net/identify) (Yoon et al., 2017). Two putative novel strains, D3-12ᵀ and G2-2ᵀ, were selected for further taxonomic analysis. These strains were routinely cultured on MA at 30°C for 2 days and preserved at -80°C in marine broth (MB; MBcell) supplemented with 15% (v/v) glycerol.
Phylogenetic analysis of 16S rRNA gene sequences and ecological distribution assessment
The 16S rRNA gene amplicons of strains D3-12ᵀ and G2-2ᵀ, initially amplified using primers 27F and 1492R, were further sequenced with universal primers 518R (5′-ATT ACC GCG GCT GCT GG-3′) and 805F (5′-GAT TAG ATA CCC TGG TAG TC-3′) (Kim et al., 2024a). Sequences obtained from primers 340F, 518R, and 805F were assembled to generate nearly complete 16S rRNA gene sequences. Sequence similarities of the 16S rRNA genes of strains D3-12ᵀ and G2-2ᵀ were compared with those of validly published type strains using the nucleotide similarity search tool on the EzBioCloud server. The 16S rRNA gene sequences of strains D3-12ᵀ and G2-2ᵀ, along with those of closely related type strains, were aligned, and phylogenetic trees were constructed using the neighbor-joining (NJ), maximum-likelihood (ML), and maximum-parsimony (MP) methods in MEGA11 software (Tamura et al., 2021), with bootstrap values calculated from 1,000 replications to assess the robustness of the phylogenetic relationships. The Kimura two-parameter model, the nearest-neighbour-interchange heuristic search method, and the pairwise deletion options were used in constructing the NJ, MP, and ML trees, respectively. Based on the 16S rRNA gene sequence similarities, Rhodalgimonas zhirmunskyi KCTC 72611T, Cognatishimia maritima KCTC 23347ᵀ, Marimonas lutisalis KCTC 62376ᵀ, Aquicoccus porphyridii KACC 18806ᵀ, Marimonas arenosa KCTC 52189ᵀ, and Ponticoccus litoralis KCCM 90028ᵀ were selected as reference strains for comparative analyses of genomic features, fatty acid composition, and phenotypic characteristics.
The potential ecological distribution of strains D3-12ᵀ and G2-2ᵀ was evaluated by comparing their 16S rRNA gene sequences with metagenomic 16S rRNA amplicon datasets from diverse environments—including seawater, freshwater, marine and freshwater sediments, oysters, corals, soil, plants, air, epibionts, and gut microbiomes—using the Integrated Microbial Next-Generation Sequencing (IMNGS) platform (Lagkouvardos et al., 2016), with a sequence similarity threshold of 99.0%.
Whole-genome sequencing, phylogenomic analysis, and assessment of genome-relatedness
For whole-genome sequencing of strains D3-12ᵀ, G2-2ᵀ, and P. litoralis KCCM 90028ᵀ, genomic DNA was extracted from cells cultured in MB using the Wizard Genomic DNA Purification Kit (Promega, USA), following the manufacturer’s protocol. The extracted genomic DNA was sequenced using the Oxford Nanopore MinION platform (ONT, UK), and the resulting sequencing reads were de novo assembled using Unicycler (version 0.4.9; Wick et al., 2017) for strain D3-12ᵀ and Flye (version 2.9.1; Kolmogorov et al., 2019) for strains G2-2ᵀ and P. litoralis KCCM 90028ᵀ. Genome completeness and contamination were assessed using CheckM2 (version 1.0.2; Chklovski et al., 2023). Phylogenomic analysis of strains D3-12ᵀ and G2-2ᵀ, along with closely related type strains, was conducted using the Genome Taxonomy Database Toolkit (GTDB-Tk) based on the concatenated protein sequences of 120 single-copy marker genes (bac120 marker set) (Chaumeil et al., 2020). Sequence alignment and construction of an ML phylogenomic tree were performed in MEGA11, with bootstrap values calculated from 1,000 replications.
Average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values between strains D3-12ᵀ, G2-2ᵀ, and their closest type strains were calculated using the Orthologous ANI Tool (OAT, version 0.93.1; www.ezbiocloud.net/tools/orthoani) (Lee et al., 2016) and the Genome-to-Genome Distance Calculator (GGDC 3.0; https://ggdc.dsmz.de/ggdc.php) using formula 2 (Meier-Kolthoff et al., 2013), respectively. Average amino acid identity (AAI) and percentage of conserved proteins (POCP) were calculated using EzAAI (version 1.2.3; Kim et al., 2021) and the method described by Qin et al. (2014), respectively.
Genomic characterization and bioinformatic analysis of algae-associated genes
The genome sequences of strains D3-12ᵀ and G2-2ᵀ and P. litoralis KCCM 90028ᵀ were submitted to GenBank and annotated using the NCBI Prokaryotic Genome Annotation Pipeline (Tatusova et al., 2016). Carbohydrate-Active Enzyme (CAZyme) genes in the genomes of strains D3-12ᵀ and G2-2ᵀ, as well as in closely related Roseobacteraceae species, were identified using the dbCAN3 meta server (https://bcb.unl.edu/dbCAN2/blast.php) with the CAZyme database as a reference (Zheng et al., 2023). In addition, the presence of metabolic genes potentially involved in symbiotic interactions with marine algae was assessed using BLASTP, by comparing reference protein sequences from the UniProt database (https://www.uniprot.org) against the genomic datasets.
Phenotypic and biochemical characterization
The growth of strains D3-12T and G2-2T was assessed on various bacteriological agar media, including MA, Reasoner’s 2A (R2A) agar, Luria-Bertani (LB) agar, tryptic soy agar (TSA), and nutrient agar (NA) (all from MBcell), each supplemented with ~2% NaCl. Cultures were incubated at 30°C for 2 days. Growth was also assessed on MA across a temperature range of 5°C to 40°C (in 5°C increments) and in MB adjusted to pH values from 4.0 to 10.0 (in 1.0 unit intervals), incubated at 30°C for 2 days. pH adjustments were made using sodium citrate (pH 4.0–5.0), sodium phosphate (pH 6.0–8.0), and sodium carbonate (pH 9.0–10.0) buffer systems, with final corrections performed after autoclaving if necessary. Salt tolerance was tested for 2 days in MB media prepared in the laboratory with varying NaCl concentrations (0% to 10% in 1% increments), following the standard MB composition. Cell morphology and motility were observed following incubation on MA at 30°C for 2 days using phase-contrast microscopy (Zeiss Axio Scope.A1; Carl Zeiss, Germany). For transmission electron microscopy (TEM), cells were placed on Formvar-coated copper grids (Electron Microscopy Sciences, USA), negatively stained with 2% (w/v) uranyl acetate for 15 s, and examined using a JEM-1010 TEM (JEOL, Japan).
Gram staining was performed using a commercial kit (bioMérieux, France) according to the manufacturer’s instructions. Catalase activity was determined by observing oxygen bubble formation in a 3% (v/v) hydrogen peroxide solution (Junsei, Japan), while oxidase activity was assessed by the oxidation of 1% (w/v) tetramethyl-p-phenylenediamine (Merck, USA), following the method described by Smibert and Krieg (1994). Anaerobic growth was evaluated on MA under anaerobic conditions using the GasPak Plus system (BBL, USA) at 30°C for 3 weeks. Phenotypic characteristics of strains D3-12ᵀ and G2-2ᵀ were compared with those of five reference strains under identical conditions at their respective optimal growth temperatures. The ability to hydrolyze esculin, casein, starch, tyrosine, Tween 20, and Tween 80 was tested on MA as previously described by Lányi (1987). Additional biochemical traits were assessed using the API 20NE system (bioMérieux), following the manufacturer’s instructions, with inocula prepared by suspending cells in ASW.
Chemotaxonomic characterization
To analyze respiratory isoprenoid quinones, strains D3-12T and G2-2T were cultivated to their exponential growth phases in MB at 30°C. Bacterial cells were harvested by centrifugation, and their respiratory isoprenoid quinones were extracted, as previously described (Minnikin et al., 1984). The extracted quinones were analyzed using a high-performance liquid chromatography system (LC-20A; Shimadzu, Japan) equipped with a diode array detector (SPD-M20A; Shimadzu) and a reversed-phase column (250 × 4.6 mm, Kromasil; Akzo Nobel). Methanol-isopropanol (2:1, v/v) was used as the eluent at a flow rate of 1 ml/min. For cellular fatty acid analysis, strains D3-12T and G2-2T, along with reference strains, were grown in MB at their optimal temperatures, and their bacterial cells were harvested at the same growth stage (exponential phase; optical density, OD600 = 0.7–0.8). The fatty acids from the microbial cells were saponified, methylated, and extracted using the standard MIDI protocol, then analyzed by gas chromatography (Hewlett Packard 6890). Cellular fatty acids were identified and quantified using the RTSBA6 database of the Microbial Identification System (Sherlock ver. 6.0B) (Sasser, 1990). Polar lipids of strains D3-12T and G2-2T were analyzed using two-dimensional thin-layer chromatography, with cells harvested during the exponential growth phase, following the method described by Minnikin et al. (1977). Different reagents were used to detect various polar lipids, including 10% ethanolic molybdophosphoric acid (for total polar lipids), ninhydrin (for aminolipids), Dittmer-Lester (for phospholipids), α-naphthol/sulfuric acid (for glycolipids), and Dragendorff (for phosphatidylcholine; PC) reagents. The presence or absence of PC, phosphatidylglycerol (PG), phosphatidylethanolamine (PE), and diphosphatidylglycerol (DPG) in strains D3-12T and G2-2T were confirmed using standard polar lipid compounds from Sigma-Aldrich (USA).
Results and Discussion
Phylogenetic characteristics of strains D3-12T and G2-2T based on 16S rRNA gene sequences
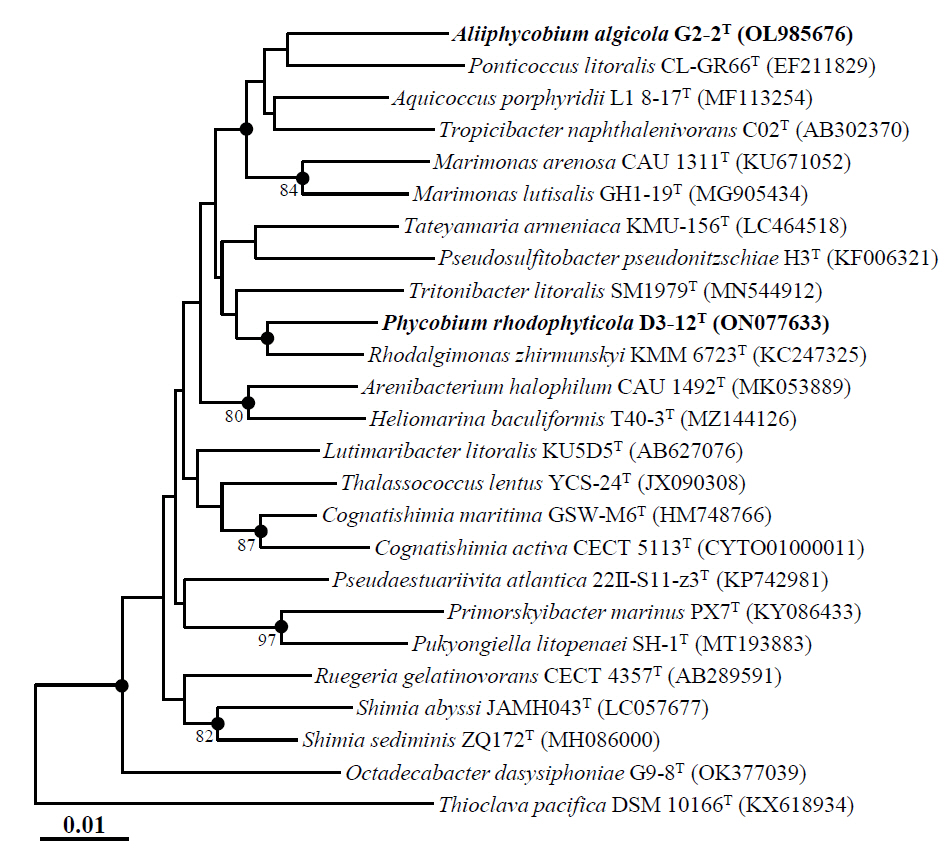
The putative novel genus strains D3-12ᵀ and G2-2ᵀ, members of the family Roseobacteraceae isolated from the phycosphere of marine algae, yielded nearly complete 16S rRNA gene sequences (1,406 bp for D3-12ᵀ and 1,401 bp for G2-2ᵀ) through sequencing and assembly of 16S rRNA gene amplicons using primers 340F, 518R, and 805F. The 16S rRNA gene sequence similarity between strains D3-12ᵀ and G2-2ᵀ was 95.6%, which is below the commonly accepted threshold of 98.5–98.7% for species delineation based on 16S rRNA gene sequences (Riesco and Trujillo, 2024), indicating that the two strains are likely distinct. Comparative sequence analysis showed that strain D3-12ᵀ was most closely related to R. zhirmunskyi KMM 6723T, C. maritima GSW-W6T, and M. lutisalis GH1-19T, with sequence similarities of 97.8%, 96.8%, and 96.5%, respectively. In contrast, strain G2-2ᵀ was most closely related to A. porphyridii L1 8-17T, M. arenosa CAU 1311T, and P. litoralis KCCM 90028T, with sequence similarities of 96.7%, 96.4%, and 96.2%, respectively. Phylogenetic analysis using the NJ algorithm showed that both strains formed distinct lineages separate from other genera within the family Roseobacteraceae, with low bootstrap values (Fig. 1). Phylogenetic trees generated using the ML and MP algorithms further confirmed the distinct placement of strains D3-12ᵀ and G2-2ᵀ from other genera within the family Roseobacteraceae (Fig. S1). Together, these comparative and phylogenetic analyses based on 16S rRNA gene sequences strongly suggest that strains D3-12ᵀ and G2-2ᵀ likely represent two novel genera within the family Roseobacteraceae.
Ecological insights of strains D3-12T and G2-2T based on their distribution
Ecological habitat distribution analysis of strains D3-12ᵀ and G2-2ᵀ using the IMNGS platform revealed that their 16S rRNA gene sequences were detected in metagenomic datasets from a wide range of environments (Table S1). Notably, their sequences were abundantly found in datasets associated with marine environments, including Panulirus ornatus, marine and seawater metagenomes, Seminavis robusta, marine sediment metagenomes, and algae, suggesting that marine ecosystems likely represent their primary ecological habitats. Interestingly, the 16S rRNA gene sequences of D3-12ᵀ and G2-2ᵀ showed differing distribution patterns across various environments, implying that although both strains were isolated from the phycosphere of marine macroalgae, they may occupy distinct ecological niches. Additionally, their sequences were also identified—though at lower abundance—in non-marine metagenomic datasets, including those from terrestrial, compost, plant, and freshwater environments, indicating a potentially broad ecological distribution, although strains D3-12ᵀ and G2-2ᵀ, as well as their closest known relatives, have been exclusively isolated from marine environments (Feng et al., 2018; Hwang and Cho, 2008; Lee et al., 2020; Park et al., 2012; Thongphrom et al., 2017).
Whole genome sequencing, phylogeny based on genome sequences, and genome relatedness
De novo assembly of MinION sequencing reads yielded complete genomes for strains D3-12T and G2-2T, with genome sizes of approximately 4,496 kb and 3,786 kb, and average genome coverages of 42.2× and 162.0×, respectively. In contrast, assembly for P. litoralis KCCM 90028T resulted in a draft genome of 4,790 kb, comprising five contigs, with an average coverage of 22.0× and an N50 value of 3,898 kb. The 16S rRNA gene sequences identified in the assembled genomes were consistent with those obtained via PCR-based sequencing. Genome quality assessment using CheckM2 indicated completeness values of 98.9%, 99.2%, and 91.1%, and contamination rates of 0.8%, 1.3%, and 1.0% for strains D3-12ᵀ, G2-2ᵀ, and P. litoralis KCCM 90028ᵀ, respectively, confirming that all genomes meet the criteria for high-quality genome assemblies (completeness ≥ 90%, contamination ≤ 10%) (Chklovski et al., 2023).
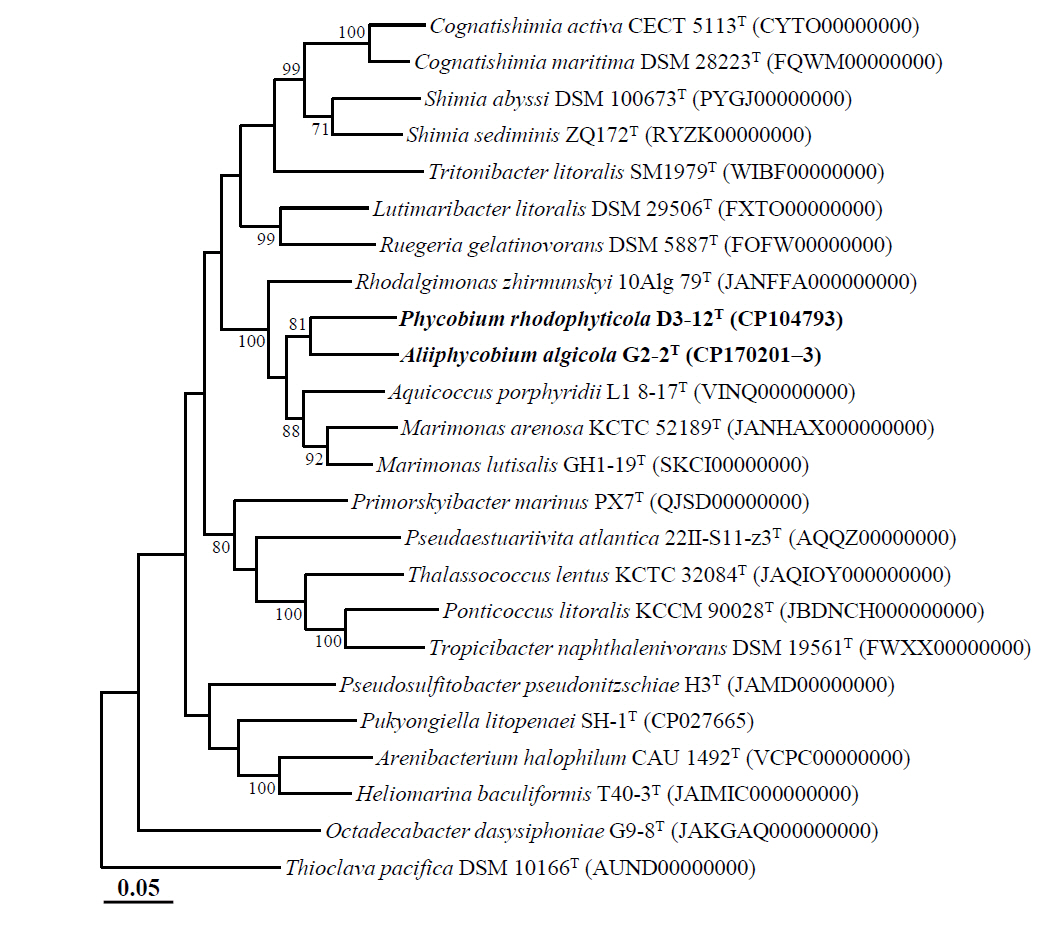
Phylogenomic analysis based on the concatenated protein sequences of 120 single-copy marker genes revealed that strains D3-12ᵀ and G2-2ᵀ clustered together within the family Roseobacteraceae (Fig. 2), in contrast to 16S rRNA gene sequence-based phylogenetic analyses, which placed them in distinct lineages (Figs. 1 and S1). Despite their phylogenomic clustering, the two strains exhibited low genome relatedness, with ANI and dDDH values of 74.5% and 18.7%, respectively (Table S2), and AAI and POCP values of 75.0% and 61.3%, respectively (Table S3). Although a genus-level POCP threshold of 50% was initially proposed for prokaryotes (Qin et al., 2014), several exceptions within the roseobacter group suggest that POCP alone may not reliably delineate genera in this group (Wirth and Whitman, 2018). Therefore, it has been suggested that POCP should be used in conjunction with other genomic indices, such as AAI, for robust genus classification. Luo et al. (2014) noted that AAI values between members of distinct genera typically range from 60% to 80%, rarely exceeding 85%. Therefore, despite clustering together in the phylogenomic tree, strains D3-12ᵀ and G2-2ᵀ represent distinct genera.
Moreover, phylogenomic analysis showed that both strains formed phylogenetic lineages clearly separated from other genera within the family Roseobacteraceae. Comparative genomic analyses with closely related genera further confirmed their distinctiveness, with ANI, dDDH, AAI, and POCP values of ≤ 75.5%, ≤ 21.0%, ≤ 76.6%, and ≤ 68.3%, respectively (Tables S2 and S3). Taken together, these results strongly support the classification of strains D3-12ᵀ and G2-2ᵀ as representatives of two distinct novel genera within the family Roseobacteraceae.
Genomic features and functional genes related to algal interactions
The complete genome of strain D3-12T consists of a single circular chromosome (4,496 kb with a G + C content of 59.9%) and encodes 4,393 genes, including 4,049 protein-coding sequences, one rRNA operon (16S, 23S, and 5S), 42 tRNA genes, and three noncoding RNA genes. Strain G2-2T has a complete genome with one circular chromosome (3,565 kb with a G + C content of 60.5%) and two plasmids (217.6 kb with 57.2% G + C content and 3.5 kb with 43.4% G + C content), encoding a total of 3,681 genes, including 3,393 protein-coding sequences, one rRNA operon (16S, 23S, and 5S), 42 tRNA genes, and three noncoding RNA genes. The draft genome of P. litoralis KCCM 90028T, consisting of five contigs (4,790 kb with a G + C content of 67.3%), contains 4,772 genes, including 3,931 protein-coding sequences, three rRNA operons (16S, 23S, and 5S), 47 tRNA genes, and three noncoding RNA genes. The G + C contents of strains D3-12T and G2-2T, based on their entire genomes, were 59.9% and 60.2%, respectively. The general genomic features of these strains, along with closely related Roseobacteraceae species, are summarized in Table 1 and show similarities to other members of the Roseobacteraceae family.
Algae are primarily composed of polysaccharides, which constitute key components of their extracellular matrices, cell walls, and storage materials. Consequently, the ability to degrade diverse algal polysaccharides is a critical trait of heterotrophic bacteria associated with marine algae (Mühlenbruch et al., 2018). Strain D3-12ᵀ encodes 52 CAZymes, including 37 glycosyltransferases (GTs)—a higher number than observed in strain G2-2ᵀ and other closely related strains (Table S4)—suggesting a broader capacity for polysaccharide utilization. Notably, the GT1 family, which encodes UDP-glucuronosyltransferases involved in the glycosylation of sugars and secondary metabolites (Ulvskov et al., 2013), was uniquely detected in strain D3-12ᵀ but not in strain G2-2ᵀ or any related strains. The presence of GT1 may indicate that strain D3-12ᵀ has the potential to modify algal-derived compounds, such as sulfated polysaccharides and polyphenols, thereby possibly enhancing their bioavailability and promoting metabolic interactions in the algal phycosphere. Additionally, carbohydrate esterase (CE) genes—responsible for removing ester-linked modifications during the initial stages of polysaccharide degradation (Li et al., 2022)—were present in D3-12ᵀ but absent in strain G2-2ᵀ (Table S4). This absence might suggest a reduced capacity for independent polysaccharide degradation in strain G2-2ᵀ, although alternative degradation mechanisms or synergistic interactions with other phycosphere bacteria cannot be excluded.
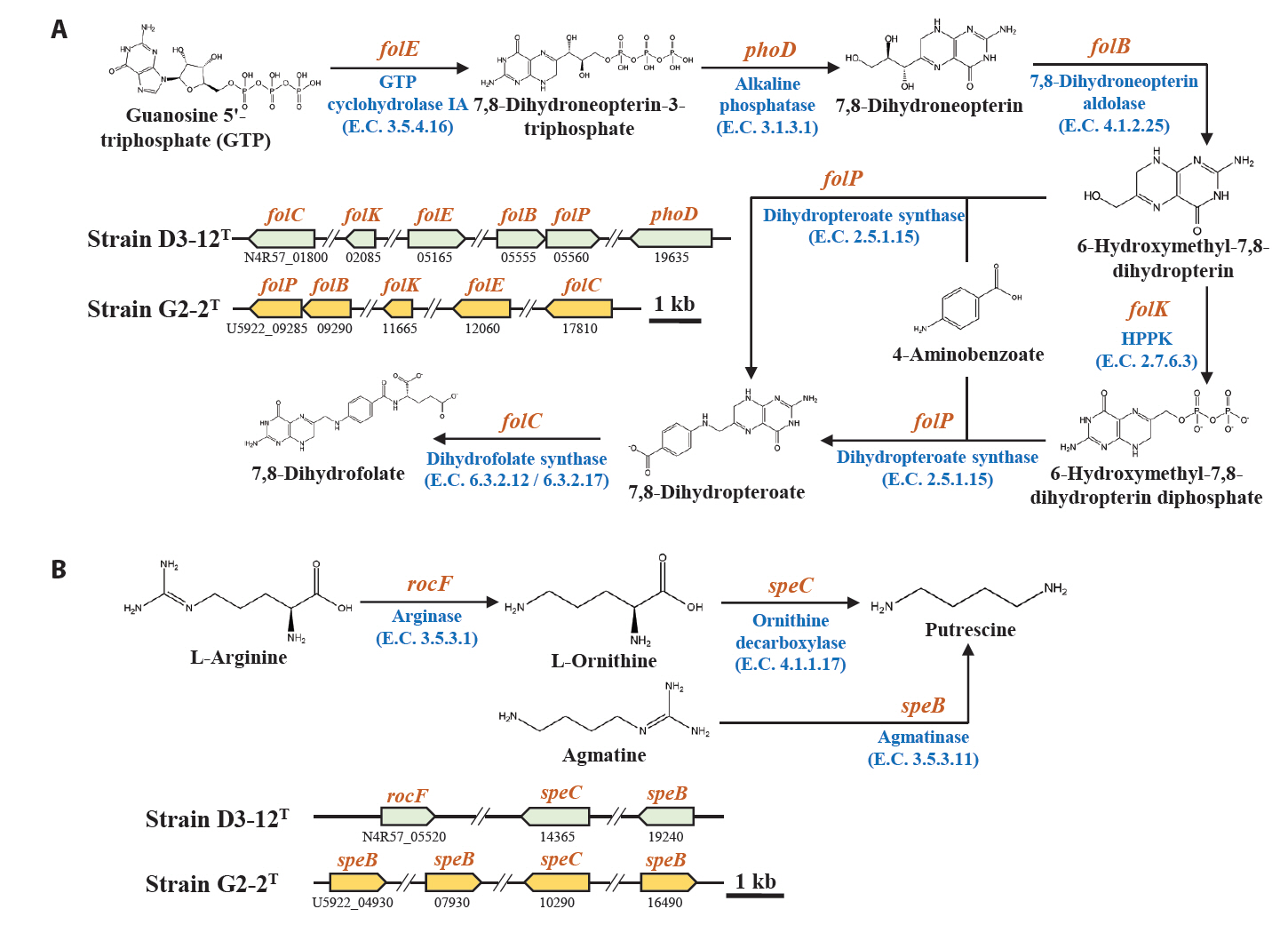
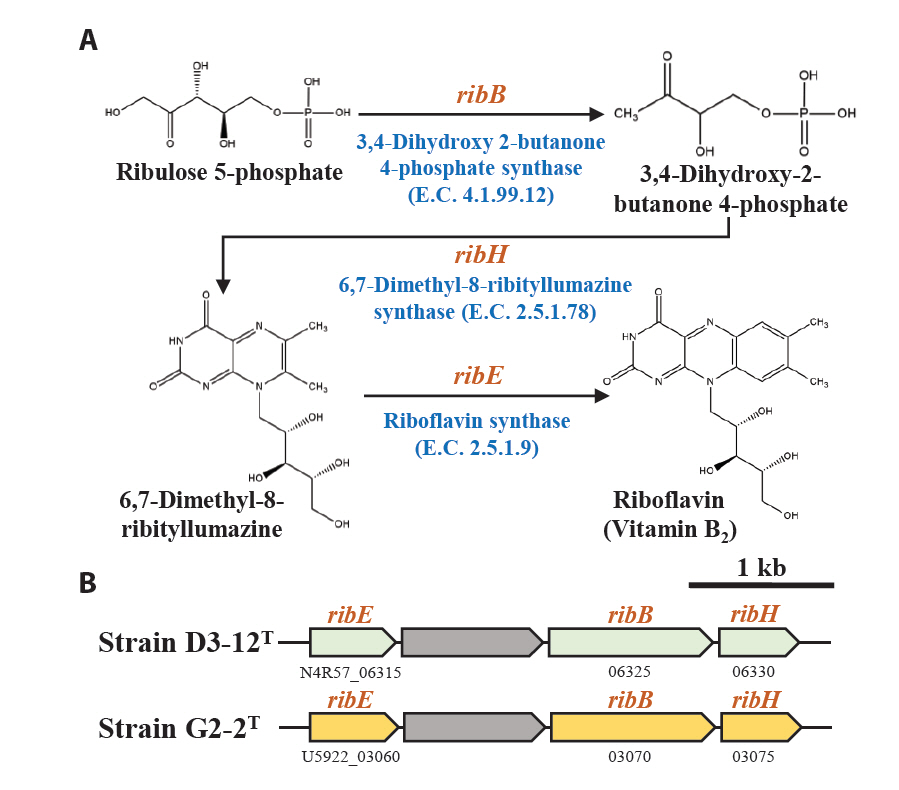
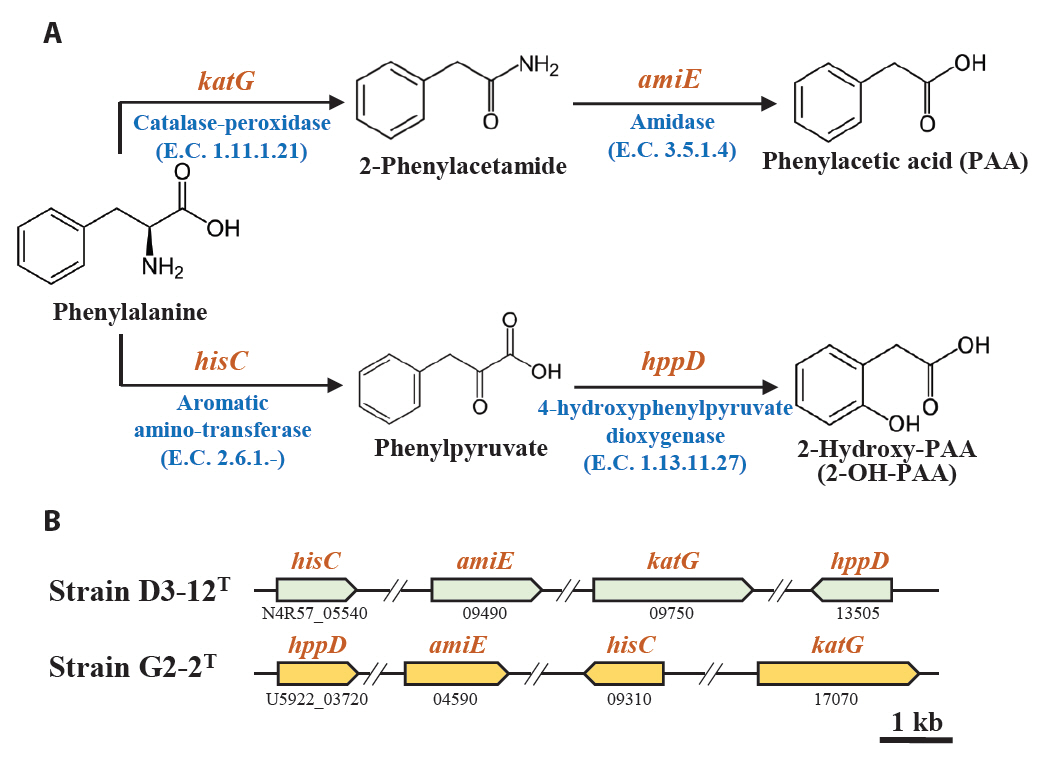
Marine bacteria in the phycosphere can influence their algal hosts through diverse metabolic interactions, including the production of vitamins, siderophores, compatible solutes, and nutrients (Kim et al., 2024b). Genomic analysis of strains D3-12ᵀ and G2-2ᵀ revealed the presence of multiple genes involved in the biosynthesis of compounds potentially beneficial to marine algal growth. Strain D3-12ᵀ possesses the complete set of genes (folBCEKP and phoD) required for the synthesis of dihydrofolate (DHF), a vitamin B9 derivative, from guanosine 5'-triphosphate (GTP). In contrast, strain G2-2ᵀ contains the folBCEKP genes but lacks phoD, which encodes alkaline phosphatase (Fig. 3A), suggesting that it may synthesize DHF from 7,8-dihydroneopterin rather than directly from GTP. Strain D3-12ᵀ also carries genes for putrescine production via two pathways: from arginine (rocF and speC) and from agmatine (speB) (Fig. 3B). Strain G2-2ᵀ, however, lacks rocF and appears capable of synthesizing putrescine only from agmatine. Additionally, only strain D3-12ᵀ harbors the cbiBP and cobASU genes involved in cobalamin (vitamin B12) biosynthesis from cobyrinate a,c-diamide, suggesting a potential role in supporting vitamin B12-dependent algal metabolism (Croft et al., 2005). Both strains possess the ribBEH gene cluster for riboflavin (vitamin B2) synthesis from ribulose-5-phosphate (Fig. 4). Furthermore, genes involved in the biosynthesis of phenylacetic acid (katG and amiE) and 2-hydroxy-phenylacetic acid (hisC and hppD) from L-phenylalanine were identified in both genomes (Fig. 5); these hormone-like compounds are known to promote algal growth and enhance stress tolerance (Kim et al., 2024b). Lastly, both strains encode bacterioferritin (bfr), a siderophore-related protein that facilitates iron acquisition and may further contribute to algal health. Collectively, these genomic features suggest that strains D3-12ᵀ and G2-2ᵀ possess metabolic traits that support symbiotic interactions with marine algae and potentially promote host growth within the phycosphere.
Phenotypic and biochemical characteristics
Strains D3-12T and G2-2T exhibited robust growth on MA. Strain D3-12T also grew well on NA supplemented with 2% NaCl, whereas strain G2-2T showed slow growth. Both strains exhibited weak growth on R2A agar and TSA (with 2% NaCl), and only strain D3-12T showed limited growth on LB agar with 2% NaCl. Cells of strains D3-12T and G2-2T were Gram-stain-negative, non-motile rods, measuring 1.1–1.2 µm wide and 2.1–2.2 µm long for strain D3-12ᵀ, and 1.0–1.1 µm wide and 2.2–2.3 µm long for strain G2-2ᵀ (Fig. S2). Neither strain exhibited anaerobic growth, confirming their strictly aerobic nature. Several phenotypic features—including aerobic metabolism, motility, catalase and oxidase activities, indole production, hydrolysis of gelatin, casein, and starch, and the assimilation of D-mannitol, capric acid, and phenylacetic acid—were consistent with characteristics of other Roseobacteraceae species (Table 2). However, strains D3-12ᵀ and G2-2ᵀ could be distinguished from closely related species by various traits, including glucose fermentation, arginine dihydrolase activity, and the assimilation of D-mannose, D-glucose, L-arabinose, and D-maltose.
Chemotaxonomic characteristics
The sole respiratory isoprenoid quinone identified in both strains D3-12ᵀ and G2-2ᵀ was ubiquinone-10 (Q-10), consistent with the predominant quinone found in other members of the family Roseobacteraceae (Coe et al., 2023; Feng et al., 2018; Lee et al., 2020; Su et al., 2024; Thongphrom et al., 2017; Yang et al., 2018, 2023). Among the major fatty acids (> 5% of total), both strains contained summed feature 8 (comprising C18:1 ω7c and/or C18:1 ω6c) and C16:0 (Table S5). In addition, strain D3-12ᵀ possessed 11-methyl-C18:1 ω7c as a major component, which was absent in strain G2-2ᵀ, suggesting a chemotaxonomic distinction between the two strains. Although the overall fatty acid profiles of D3-12ᵀ and G2-2ᵀ were similar to those of closely related Roseobacteraceae species, notable differences were observed in the relative abundance of 1-methyl-C18:1 ω7c, C18:0, and cyclo-C19:0 ω8c (Table S5). The major polar lipids in strain D3-12ᵀ included PG, PE, and PC, along with an unidentified aminolipid and three unidentified lipids. In contrast, strain G2-2ᵀ contained PG and DPG, an unidentified aminolipid, and two unidentified lipids (Fig. S3). The presence or absence of PC, PE, and DPG between the two strains further supports their differentiation at the genus level. Nonetheless, both strains exhibited polar lipid profiles generally consistent with other Roseobacteraceae species (Table 2).
Taxonomic conclusion
Based on phylogenetic analyses of 16S rRNA gene and whole-genome sequences, along with physiological and chemotaxonomic characteristics, strains D3-12ᵀ and G2-2ᵀ are proposed to represent two novel and distinct genera within the family Roseobacteraceae. Accordingly, we propose the names Phycobium rhodophyticola gen. nov., sp. nov. for strain D3-12ᵀ, and Aliiphycobium algicola gen. nov., sp. nov. for strain G2-2ᵀ.
Description of Phycobium gen. nov.
Phycobium (Phy.co′bi.um. Gr. neut. n. phykos, seaweed; Gr. masc. n. bios, life; N.L. neut. n. Phycobium, a living form from an alga).
Cells are Gram-stain-negative, strictly aerobic, and non-motile rods. Oxidase and catalase activities are positive. Nitrate is not reduced to nitrite. Q-10 is identified as the sole respiratory quinone. The major cellular fatty acids (> 5%) are summed feature 8 (C18:1 ω7c and/or C18:1 ω6c), 11-methyl-C18:1 ω7c, and C16:0. The major polar lipids are PC, PG, and PE. Phylogenetically, the genus is a member of the family Roseobacteraceae within the order Rhodobacterales of the phylum Pseudomonadota. The type species is Phycobium rhodophyticola.
Description of Phycobium rhodophyticola sp. nov.
Phycobium rhodophyticola (rho.do.phy.ti′co.la. N.L. neut. pl. n. Rhodophyta, the division of the red algae; L. suffix. -cola (from L. masc. or fem. n. incola), inhabitant, dweller; N.L. masc. n. rhodophyticola, inhabitant of Rhodophyta).
In addition to the characteristics described for the genus, this species exhibits the following traits. Colonies grown on MA are smooth and round. Growth occurs between 10–35°C (optimal at 30°C) and pH 6.0–9.0 (optimal at pH 7.0), with NaCl concentrations of 1.0–6.0% (w/v) (optimal at 2.0–3.0%). Esculin hydrolysis is positive, while hydrolysis of tyrosine, casein, starch, gelatin, Tween 20, and Tween 80 is negative. Fermentation of D-glucose is positive, but indole production is negative. Positive for arginine dihydrolase, urease, and β-galactosidase activities. Assimilates N-acetyl-glucosamine, trisodium citrate, and malic acid, but not D-glucose, L-arabinose, D-maltose, D-mannose, D-mannitol, potassium gluconate, adipic acid, capric acid, and phenylacetic acid.
The type strain is D3-12T (= KACC 22712T = JCM 35528T), isolated from the phycosphere of the marine red alga Melanothamnus japonicus, collected from a coastal region in Korea. The genome size of the strain is 4,496 kb, and its DNA G + C content is 59.9%, as determined from the whole genome sequence. The GenBank accession numbers for the 16S rRNA gene and the genome sequences of strain D3-12T are ON077633 and CP104793, respectively.
Description of Aliiphycobium gen. nov.
Aliiphycobium (A.li.i.phy.co′bi.um. L. masc. adj. alius, other, another; N.L. masc. n. Phycobium, a bacterial generic name; N.L. masc. n. Aliiphycobium, the other Phycobium).
Cells are Gram-stain-negative, strictly aerobic, and non-motile rods without flagella. Oxidase and catalase activities are positive. Nitrate is reduced to nitrite. Q-10 is identified as the sole respiratory quinone. The major cellular fatty acids are summed feature 8 (C18:1 ω7c and/or C18:1 ω6c) and C16:0. The major polar lipids are PG and DPG. Phylogenetically, the genus is a member of the family Roseobacteraceae within the order Rhodobacterales of the phylum Pseudomonadota. The type species is Aliiphycobium algicola.
Description of Aliiphycobium algicola sp. nov.
Aliiphycobium algicola, (al.gi′co.la. L. fem. n. alga, an alga; L. suffix. -cola (from L. masc. or fem. n. incola), inhabitant, dweller; N.L. masc. or fem. n. algicola, an alga dweller).
In addition to the characteristics described for the genus, this species exhibits the following traits. Colonies grown on MA are circular and smooth. Growth occurs between 10–35°C (optimal at 30°C) and pH 6.0–8.0 (optimal at pH 7.0), with NaCl concentrations of 1.0–6.0% (w/v) (optimal at 2.0%). Hydrolysis of esculin, tyrosine, casein, starch, gelatin, Tween 20, and Tween 80 is negative. Fermentation of D-glucose is positive, but indole production is negative. Positive for arginine dihydrolase activity, but negative for urease and β-galactosidase activities. Assimilates adipic acid, but not D-glucose, L-arabinose, D-maltose, D-mannose, D-mannitol, N-acetyl-glucosamine, trisodium citrate, potassium gluconate, malic acid, capric acid and phenylacetic acid.
The type strain is G2-2T (= KACC 22602T = JCM 35752T), isolated from the phycosphere of the marine red alga Chondrus species, collected from a coastal region in Korea. The genome size of the strain is 3,786 kb, and its DNA G + C content is 60.2%, as determined from the whole genome sequence. The GenBank accession numbers for the 16S rRNA gene and the genome sequences of strain G2-2T are OL985676 and CP170201– CP170203, respectively.
Acknowledgments
This work was supported by the Chung-Ang University Research Grants in 2023 and the Marine Biotics project (20210469) funded by the Ministry of Ocean and Fisheries, Republic of Korea. We also thank Dr. Aharon Oren (The Hebrew University of Jerusalem, Israel) for his etymological advice.
Conflict of Interest
The authors declare no competing financial conflicts of interest.
Data Availability
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequences of strains D3-12T and G2-2T are ON077633 and OL985676, respectively, and those for the genome sequences of strain D3-12T, strain G2-2T, and Ponticoccus litoralis KCCM 90028T are CP104793, CP170201–3, and JBDNCH000000000, respectively.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.71150/jm.2503014.
Fig. S1.
Maximum-likelihood (A) and maximum-parsimony (B) trees showing the phylogenetic relationships between strains D3-12T and G2-2T and their closely related taxa, based on 16S rRNA gene sequences. Only bootstrap values exceeding 70% are indicated on the nodes as percentages from 1000 replicates. Thioclava pacifica DSM 10166T (KX618934) was used as an outgroup. The scale bars in panels A and B indicate nucleotide changes per nucleotide and throughout the entire sequence, respectively.
jm-2503014-Supplementary-Fig-S1.pdf
Fig. S2.
Transmission electron micrographs of negatively stained (using 2% uranyl acetate) cells showing the general morphologies of strains D3-12T (A) and G2-2T (B) grown on marine agar at 30°C for 2 days.
jm-2503014-Supplementary-Fig-S2.pdf
Fig. S3.
Two-dimensional thin-layer chromatograms (TLC) showing the polar lipids profiles of strains D3-12T and G2-2T. Solvent systems: (I) chloroform-methanol-water (65:25:4, v/v/v) and (II) chloroform-acetic acid-methanol-water (80:15:12:4, v/v/v/v). The TLC plates were sprayed with 10% ethanolic molybdophosphoric acid (A), ninhydrin (B), Dittmer-Lester (C) and Dragendorff (D) reagents for the detection of total polar lipids, aminolipids, phospholipids, and phosphatidylcholine, respectively. The TLC plates for glycolipid detection using α-naphthol/sulfuric acid reagent were not displayed, as no spots were observed. Abbreviations: PC, phosphatidylcholine; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; DPG, diphosphatidylglycerol; AL, unidentified aminolipid; L, unidentified lipid.
jm-2503014-Supplementary-Fig-S3.pdf
Table S1.
Potential ecological distribution of strains D3-12T and G2-2T assessed by comparing their 16S rRNA gene sequences against metagenomic 16S rRNA amplicon datasets using the IMNGS platform, with a sequence similarity threshold of 99.0%. “Matched No.” indicates the number of metagenomic datasets including sequences matching the 16S rRNA gene of each strain, while “ARA” represents the average relative abundance of these sequences within the respective datasets. ARA values below 0.05% for both strains in all datasets were excluded.
jm-2503014-Supplementary-Table-S1.pdf
Table S2.
Average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values among strains D3-12T and G2-2T and their closely related taxa of the family Roseobacteraceae
jm-2503014-Supplementary-Table-S2.pdf
Table S3.
Average amino acid identity (AAI) and percentage of conserved proteins (POCP) among strains D3-12T and G2-2T and their closely related taxa of the family Roseobacteraceae
jm-2503014-Supplementary-Table-S3.pdf
Table S4.
Profiles of carbohydrate-active enzyme (CAZyme) genes identified in the genomes of strains D3-12T and G2-2T, along with those of their closely related type strains
jm-2503014-Supplementary-Table-S4.pdf
Fig. 1.Neighbor-joining tree showing the phylogenetic relationships between strains D3-12T and G2-2T and their closely related type strains, based on 16S rRNA gene sequences. Numbers at the nodes represent bootstrap percentages from 1,000 replicates; only values greater than 70% are shown. Filled circles (●) mark nodes that were also supported in the maximum-likelihood and maximum-parsimony trees. Thioclava pacifica DSM 10166T (KX618934) was employed as the outgroup. Scale bar represents 0.01 nucleotide substitutions per site.
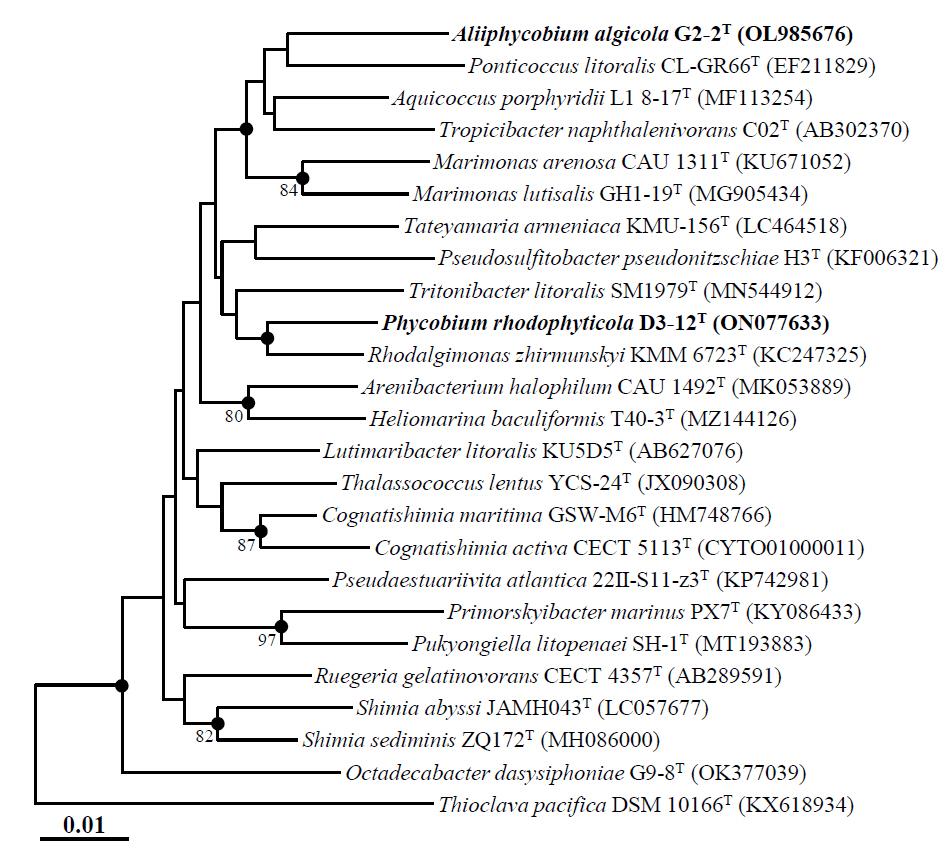
Fig. 2.Phylogenomic tree showing the phylogenetic relationships between strains D3-12T and G2-2T and their closely related type strains, based on the concatenated protein sequences of 120 ubiquitous single-copy marker genes (bac120 marker set) of GTDB-Tk. Numbers at the nodes represent bootstrap percentages from 1,000 replicates; only values greater than 70% are shown. Thioclava pacifica DSM 10166T (AUND00000000) was employed as the outgroup. Scale bar represents 0.05 substitutions per amino acid.
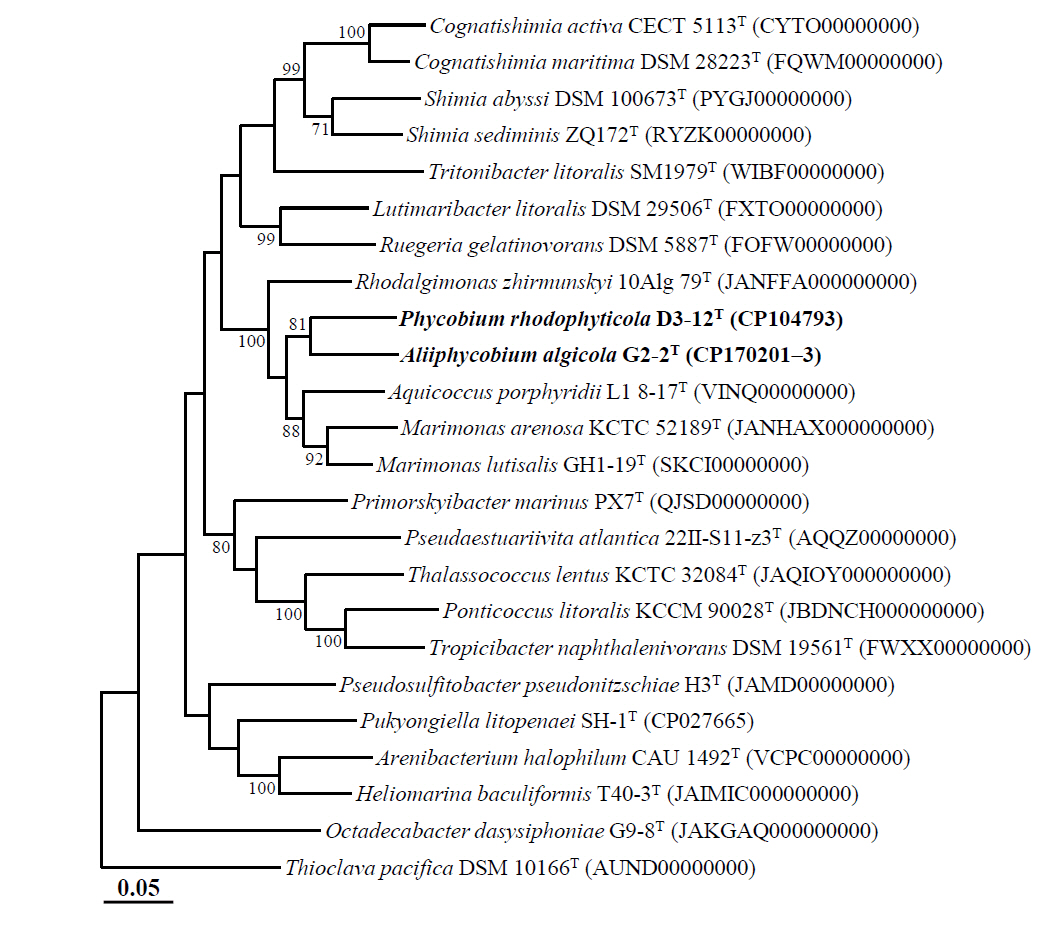
Fig. 3.Biosynthetic pathways and associated genes for the biosynthesis of 7,8-dihydrofolate from GTP (A) and putrescine from L-arginine or agmatine (B) identified in strains D3-12T and G2-2T. HPPK, 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase.
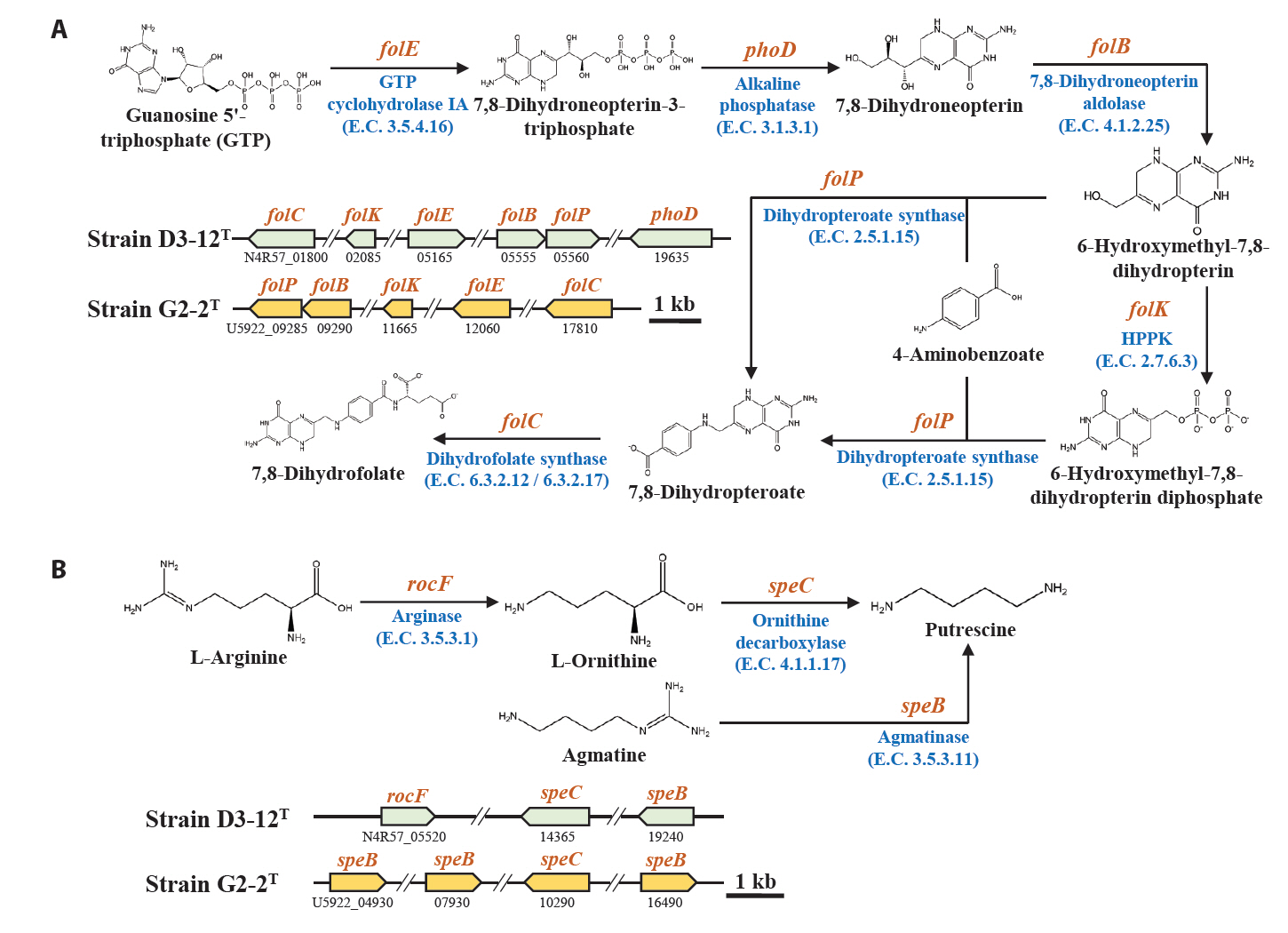
Fig. 4.Metabolic pathway (A) and associated gene clusters (B) for the biosynthesis of the riboflavin from ribulose 5-phosphate identified in strains D3-12T and G2-2T.
Fig. 5.Metabolic pathway (A) and associated genes (B) for the biosynthesis of phenylacetic acid (PAA) and 2-hydroxy-PAA (2-OH-PAA) from phenylalanine identified in strains D3-12T and G2-2T.
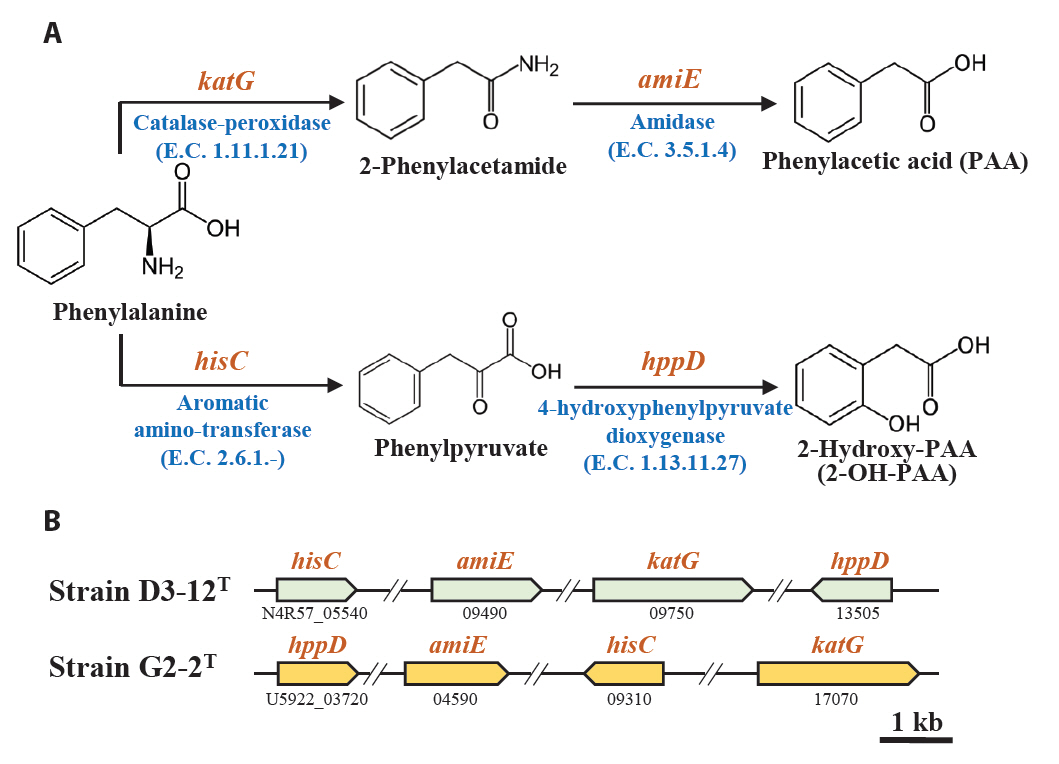
Table 1.General genomic features† of strains D3-12T and G2-2T and their closely related taxa of the family Roseobacteraceae. Taxa: 1, strain D3-12T (CP104793); 2, strain G2-2T (CP170201–3); 3, Rhodalgimonas zhirmunskyi 10Alg 79T (JANFFA000000000); 4, Ponticoccus litoralis KCCM 90028T (JBDNCH000000000); 5, Aquicoccus porphyridii L1 8-17T (VINQ00000000); 6, Marimonas lutisalis GH1-19T (SKCI00000000); 7, Marimonas arenosa KCTC 52189T (JANHAX000000000); 8, Cognatishimia maritima DSM 28223T (FQWM00000000)
|
Feature |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
Genome status (no. of contigs)‡
|
C (1) |
C (3) |
D (73) |
D (5) |
D (256) |
D (14) |
D (38) |
D (14) |
|
Genome size (kb) |
4,496 |
3,786 |
3,755 |
4,790 |
4,514 |
4,320 |
4,381 |
3,285 |
|
G + C content (%) |
59.9 |
60.2 |
62.1 |
67.3 |
63.2 |
63.2 |
63.3 |
56.3 |
|
No. of total genes |
4,393 |
3,681 |
3,626 |
4,772 |
4,560 |
4,260 |
4,277 |
3,304 |
|
No. of protein-coding genes |
4,049 |
3,393 |
3,548 |
3,931 |
4,379 |
4,151 |
4,186 |
3,214 |
|
No. of total RNA genes |
48 |
48 |
48 |
59 |
52 |
50 |
50 |
65 |
|
No. of tRNA genes |
42 |
42 |
41 |
47 |
43 |
44 |
44 |
50 |
|
No. of rRNA (16S, 23S, 5S) operons |
1 |
1 |
1 |
3 |
2 |
1 |
1 |
4 |
|
No. noncoding RNA genes |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
Table 2.Differential phenotypic characteristics between strains D3-12T and G2-2T and their closely related taxa of the family Roseobacteraceae. Taxa: 1, strain D3-12T (this study); 2, strain G2-2T; 3, Rhodalgimonas zhirmunskyi KCTC 72611T (Nedashkovskaya et al., 2023); 4, Ponticoccus litoralis KCCM 90028T (Hwang and Cho, 2008); 5, Aquicoccus porphyridii KACC 18806T (Feng et al., 2018); 6, Marimonas lutisalis KCTC 62376T (Lee et al., 2020); 7, Marimonas arenosa KCTC 52189T (Thongphrom et al., 2017); 8, Cognatishimia maritima KCTC 23347T (Park et al., 2012). All strains are positive for the following characteristics: activity* of catalase and oxidase. All strains are negative for the following characteristics: Gram-staining, anaerobic growth, indole production*, hydrolysis* of gelatin and assimilation* of capric acid. Symbols: +, positive; –, negative; w, weakly positive
|
Characteristic |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
Isolation source |
Marine alga |
Marine alga |
Marine alga |
Coastal seawater |
Marine alga |
Tidal mudflat |
Sea sand |
Seawater |
|
Colony color |
Beige |
Beige |
Beige |
Cream |
Beige*
|
Beige |
Beige |
Cream |
|
Cell morphology |
Rod |
Rod |
Rod |
Coccus |
Coccus |
Rod |
Rod |
Rod |
|
Cell motility |
– |
– |
– |
– |
– |
+ |
– |
+ |
|
Range for growth: |
|
Temperature (°C) |
10–35 |
10–35 |
4–40 |
10–37 |
20–40 |
10–40 |
20–37 |
10–37 |
|
pH |
6.0–9.0 |
6.0–8.0 |
6.0–9.0 |
6.0–8.0 |
6.0–10.0 |
6.0–9.0 |
6.5–10.0 |
6.0–8.0 |
|
NaCl (%, w/v) |
1–6 |
1–6 |
0–5 |
1–15 |
0–7 |
1–9 |
0–6 |
0–7 |
|
Nitrate reduction*
|
– |
+ |
– |
+ |
– |
– |
+ |
– |
|
Glucose fermentation*
|
+ |
+ |
– |
+ |
– |
– |
– |
– |
|
Hydrolysis* of: |
|
Casein |
– |
– |
– |
– |
– |
– |
– |
+ |
|
Esculin |
+ |
– |
w
|
+ |
– |
– |
– |
– |
|
Starch |
– |
– |
w
|
– |
– |
– |
– |
– |
|
Tween 20, Tween 80 |
– |
– |
+ |
+ |
– |
– |
– |
– |
|
Tyrosine |
– |
– |
– |
+ |
+ |
– |
– |
+ |
|
Enzyme activity* of: |
|
Arginine dihydrolase |
+ |
+ |
– |
+ |
– |
– |
– |
– |
|
Urease |
+ |
– |
– |
+ |
– |
– |
– |
– |
|
β-Galactosidase |
w
|
– |
– |
+ |
+ |
– |
– |
– |
|
Assimilation* of: |
|
D-Glucose |
– |
– |
+ |
w
|
+ |
– |
– |
+ |
|
L-Arabinose, D-maltose |
– |
– |
+ |
+ |
– |
+ |
w
|
– |
|
D-Mannose |
– |
– |
+ |
w
|
+ |
+ |
w
|
+ |
|
D-Mannitol |
– |
– |
+ |
– |
– |
– |
– |
+ |
|
N-Acetyl-glucosamine |
w
|
– |
+ |
– |
– |
– |
– |
– |
|
Trisodium citrate |
w
|
– |
– |
– |
– |
– |
– |
– |
|
Potassium gluconate |
– |
– |
+ |
+ |
– |
– |
+ |
– |
|
Phenylacetic acid |
– |
– |
+ |
– |
– |
– |
– |
– |
|
Adipic acid |
– |
w
|
+ |
w
|
– |
– |
+ |
– |
|
Malic acid |
+ |
– |
+ |
+ |
+ |
+ |
w
|
+ |
|
Major polar lipids†
|
PC, PG, PE |
PG, DPG |
PC, PG, PE |
PC, PG, PE |
PC, PG, PE |
PC, PG, PE, DPG |
PC, PG, PE |
PC, PG, PE |
References
- Bayburt H, Choi BJ, Kim JM, Baek JH, Jeon CO. 2024. Psychrosphaera algicola sp. nov. and Paraglaciecola algarum sp. nov., and reclassification of Pseudoalteromonas elyakovii, Pseudoalteromonas flavipulchra, and Pseudoalteromonas profundi as later heterotypic synonyms of P. distincta, P. maricaloris, and P. gelatinilytica. Int J Syst Evol Microbiol. 74: 006491.ArticlePubMed
- Chaumeil PA, Mussig AJ, Hugenholtz P, Parks DH. 2020. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 36: 1925–1927. ArticlePDF
- Chklovski A, Parks DH, Woodcroft BJ, Tyson GW. 2023. CheckM2: a rapid, scalable and accurate tool for assessing microbial genome quality using machine learning. Nat Methods. 20: 1203–1212. ArticlePubMedPDF
- Cirri E, Pohnert G. 2019. Algae-bacteria interactions that balance the planktonic microbiome. New Phytol. 223: 100–106. ArticlePubMedPDF
- Coe LS, Fei C, Weston J, Amin SA. 2023. Phycobacter azelaicus gen. nov. sp. nov., a diatom symbiont isolated from the phycosphere of Asterionellopsis glacialis. Int J Syst Evol Microbiol. 73: 006104.Article
- Crenn K, Serpin D, Lepleux C, Overmann J, Jeanthon C. 2016. Silicimonas algicola gen. nov., sp. nov., a member of the Roseobacter clade isolated from the cell surface of the marine diatom Thalassiosira delicatula. Int J Syst Evol Microbiol. 66: 4580–4588. ArticlePubMed
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 438: 90–93. ArticlePubMedPDF
- Cui H, Wang S, Fan S, Long H, Lin J, et al. 2025. Branched-chain amino acid metabolism supports Roseobacteraceae positive interactions in marine biofilms. Appl Environ Microbiol. 91: e02411–24. ArticlePubMedPMCPDF
- Ding W, Wang S, Qin P, Fan S, Su X, et al. 2023. Anaerobic thiosulfate oxidation by the Roseobacter group is prevalent in marine biofilms. Nat Commun. 14: 2033.ArticlePubMedPMCPDF
- Evans RD, Wilson SK, Field SN, Moore JAY. 2014. Importance of macroalgal fields as coral reef fish nursery habitat in north-west Australia. Mar Biol. 161: 599–607. ArticlePDF
- Feng T, Kim KH, Jeong SE, Kim W, Jeon CO. 2018. Aquicoccus porphyridii gen. nov., sp. nov., isolated from a small marine red alga, Porphyridium marinum. Int J Syst Evol Microbiol. 68: 283–288. ArticlePubMed
- Geng H, Belas R. 2010. Molecular mechanisms underlying Roseobacter-phytoplankton symbioses. Curr Opin Biotechnol. 21: 332–338. ArticlePubMed
- Gosink JJ, Herwig RP, Staley JT. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst Appl Microbiol. 20: 356–365. Article
- Guiry MD. 2024. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J Phycol. 60: 214–228. ArticlePubMed
- Han DM, Jeon JH, Jin MS, Choi DG, Kim JM, et al. 2024. Yoonia algicola sp. nov., Yoonia rhodophyticola sp. nov. and Yoonia phaeophyticola sp. nov., isolated from marine algae. Int J Syst Evol Microbiol. 74: 006545.ArticlePubMedPMC
- Hwang CY, Cho BC. 2008. Ponticoccus litoralis gen. nov., sp. nov., a marine bacterium in the family Rhodobacteraceae. Int J Syst Evol Microbiol. 58: 1332–1338. ArticlePubMed
- Jin MS, Kim KH, Baek JH, Kim JM, Jeon CO. 2023. Octadecabacter algicola sp. nov. and Octadecabacter dasysiphoniae sp. nov., isolated from a marine red alga and emended description of the genus Octadecabacter. Int J Syst Evol Microbiol. 73: 005664.Article
- Jung HS, Jeong SE, Chun BH, Quan ZX, Jeon CO. 2019. Rhodophyticola porphyridii gen. nov., sp. nov., isolated from a red alga, Porphyridium marinum. Int J Syst Evol Microbiol. 69: 1656–1661. ArticlePubMed
- Kim JM, Baek W, Choi BJ, Bayburt H, Baek JH, et al. 2024a. Devosia rhodophyticola sp. nov. and Devosia algicola sp. nov., isolated from a marine red alga. Int J Syst Evol Microbiol. 74: 006223.Article
- Kim KH, Kim JM, Baek JH, Jeong SE, Kim H, et al. 2024b. Metabolic relationships between marine red algae and algae-associated bacteria. Mar Life Sci Technol. 6: 298–314. ArticlePDF
- Kim D, Park S, Chun J. 2021. Introducing EzAAI: a pipeline for high throughput calculations of prokaryotic average amino acid identity. J Microbiol. 59: 476–480. ArticlePubMedPDF
- Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 37: 540–546. ArticlePubMedPDF
- Lagkouvardos I, Joseph D, Kapfhammer M, Giritli S, Horn M, et al. 2016. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep. 6: 33721.ArticlePubMedPMCPDF
- Lane DJ. 1991. 16S/23S rRNA sequencing. In Stackebrandt E, Goodfellow M. (eds.), Nucleic acid techniques in bacterial systematics, pp. 115–175. Wiley.PDF
- Lányi B. 1987. 1 Classical and rapid identification methods for medically important bacteria. Methods Microbiol. 19: 1–67. Article
- Lee MW, Baek JH, Kim JM, Bayburt H, Choi BJ, et al. 2024a. Roseovarius phycicola sp. nov. and Roseovarius rhodophyticola sp. nov., isolated from marine red algae. Int J Syst Evol Microbiol. 74: 006574.Article
- Lee JK, Choi DG, Choi BJ, Kim JM, Jeon CO. 2024b. Coraliomargarita algicola sp. nov., isolated from a marine green alga. Int J Syst Evol Microbiol. 74: 006367.Article
- Lee SD, Jeon D, Kim YJ, Kim IS, Choe H, et al. 2020. Marimonas lutisalis sp. nov., isolated from a tidal mudflat and emended description of the genus Marimonas. Int J Syst Evol Microbiol. 70: 259–266. ArticlePubMed
- Lee MW, Kim JM, Kim KH, Choi DG, Lee JK, et al. 2024c. Roseibium algicola sp. nov. and Roseibium porphyridii sp. nov., isolated from marine red algae. Int J Syst Evol Microbiol. 74: 006283.Article
- Lee I, Kim YO, Park SC, Chun J. 2016. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 66: 1100–1103. ArticlePubMed
- Li J, He Z, Liang Y, Peng T, Hu Z. 2022. Insights into algal polysaccharides: a review of their structure, depolymerases, and metabolic pathways. J Agric Food Chem. 70: 1749–1765. ArticlePubMed
- Liang KY, Orata FD, Boucher YF, Case RJ. 2021. Roseobacters in a sea of poly-and paraphyly: whole genome-based taxonomy of the family Rhodobacteraceae and the proposal for the split of the “Roseobacter clade” into a novel family, Roseobacteraceae fam. nov. Front Microbiol. 12: 683109.ArticlePubMedPMC
- Luo C, Rodriguez-r LM, Konstantinidis KT. 2014. MyTaxa: an advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res. 42: e73. ArticlePubMedPMC
- Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 14: 60.ArticlePMCPDF
- Minnikin DE, O'Donnell AG, Goodfellow M, Alderson G, Athalye M, et al. 1984. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods. 2: 233–241. Article
- Minnikin DE, Patel PV, Alshamaony L, Goodfellow M. 1977. Polar lipid composition in the classification of Nocardia and related bacteria. Int J Syst Evol Microbiol. 27: 104–117. Article
- Mühlenbruch M, Grossart HP, Eigemann F, Voss M. 2018. Mini‐review: Phytoplankton‐derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environ Microbiol. 20: 2671–2685. ArticlePubMedPDF
- Nedashkovskaya O, Otstavnykh N, Balabanova L, Bystritskaya E, Kim SG, et al. 2023. Rhodoalgimonas zhirmunskyi gen. nov., sp. nov., a marine alphaproteobacterium isolated from the Pacific red alga Ahnfeltia tobuchiensis: phenotypic characterization and pan-genome analysis. Microorganisms. 11: 2463.ArticlePubMedPMC
- Park S, Lee MH, Lee JS, Oh TK, Yoon JH. 2012. Thalassobius maritimus sp. nov., isolated from seawater. Int J Syst Evol Microbiol. 62: 8–12. ArticlePubMed
- Qin QL, Xie BB, Zhang XY, Chen XL, Zhou BC, et al. 2014. A proposed genus boundary for the prokaryotes based on genomic insights. J Bacteriol. 196: 2210–2215. ArticlePubMedPMCPDF
- Riesco R, Trujillo ME. 2024. Update on the proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 74: 006300.ArticlePubMedPMC
- Sasser M. 1990. Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI Inc., USA.PDF
- Smibert RM, Krieg NR. 1994. Phenotypic characterization. In Gerhardt P, Murray RGE, Wood WA, Krieg NR. (eds.), Methods for general and molecular bacteriology, pp. 607–654. ASM Press.PDF
- Su Z, Xu Y, Xiao Y, Chen B, Qiu X, et al. 2024. Mesobacterium hydrothermale sp. nov., isolated from shallow-sea hydrothermal systems off Kueishantao Island. Antonie van Leeuwenhoek. 117: 93.ArticlePubMedPDF
- Tak H, Park MS, Cho H, Lim Y, Cho JC. 2024. Congregibacter variabilis sp. nov. and Congregibacter brevis sp. nov. within the OM60/NOR5 clade, isolated from seawater, and emended description of the genus Congregibacter. J Microbiol. 62: 739–748. ArticlePubMedPDF
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38: 3022–3027. ArticlePubMedPMCPDF
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, et al. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44: 6614–6624. ArticlePubMedPMC
- Thongphrom C, Kim JH, Yoon JH, Bora N, Kim W. 2017. Marimonas arenosa gen. nov., sp. nov., isolated from sea sand. Int J Syst Evol Microbiol. 67: 121–126. ArticlePubMed
- Ulvskov P, Paiva DS, Domozych D, Harholt J. 2013. Classification, naming and evolutionary history of glycosyltransferases from sequenced green and red algal genomes. PLoS One. 8: e76511. ArticlePubMedPMC
- Wang YH, Liu JC, Du YH, Xu JH, Du ZJ, et al. 2023. Psychromarinibacter sediminicola sp. nov., a novel moderately halophilic, metabolically diverse bacterium isolated from a solar saltern sediment, and comparison between members of family Roseobacteraceae. Arch Microbiol. 205: 331.ArticlePubMedPDF
- Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, et al. 2017. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 29: 949–982. ArticlePubMedPDF
- Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 13: e1005595. ArticlePubMedPMC
- Wirth JS, Whitman WB. 2018. Phylogenomic analyses of a clade within the roseobacter group suggest taxonomic reassignments of species of the genera Aestuariivita, Citreicella, Loktanella, Nautella, Pelagibaca, Ruegeria, Thalassobius, Thiobacimonas and Tropicibacter, and the proposal of six novel genera. Int J Syst Evol Microbiol. 68: 2393–2411. ArticlePubMed
- Yang P, Liang J, Yin Q, Li G, Zhang Y, et al. 2023. Pacificoceanicola onchidii gen. nov., sp. nov., isolated from a marine invertebrate from the South China Sea. Int J Syst Evol Microbiol. 73: 006103.Article
- Yang SH, Park MJ, Oh HM, Park YJ, Kwon KK. 2024. Flavivirga spongiicola sp. nov. and Flavivirga abyssicola sp. nov., isolated from marine environments. J Microbiol. 62: 11–19. ArticlePubMedPDF
- Yang Q, Zhang X, Li L, Zhang R, Feng L, et al. 2018. Ponticoccus alexandrii sp. nov., a novel bacterium isolated from the marine toxigenic dinoflagellate Alexandrium minutum. Antonie van Leeuwenhoek. 111: 995–1000. ArticlePubMedPDF
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, et al. 2017. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 67: 1613–1617. ArticlePubMedPMC
- Zheng J, Ge Q, Yan Y, Zhang X, Huang L, et al. 2023. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 51: W115–W121. ArticlePubMedPMCPDF
Citations
Citations to this article as recorded by

- Ningiella algicola sp. nov. and Marinicella algicola sp. nov., proposal of Paralteromonas gen. Nov. and Neoalteromonas gen. Nov. with reclassification of Alteromonas species, and reclassification of Methylophaga aminisulfidivorans as a later heterotypic s
Hülya Bayburt, Jeong Min Kim, Byeong Jun Choi, Jae Kyeong Lee, Che Ok Jeon
Systematic and Applied Microbiology.2026; 49(1): 126685. CrossRef -
Mucilaginibacter aureus sp. nov. and Mucilaginibacter sediminis sp. nov., isolated from wetland soil
Chae Yeong Moon, Jae Kyeong Lee, Dong Min Han, Dae Seung Lee, Byeong Jun Choi, Ju Hye Baek, Che Ok Jeon
International Journal of Systematic and Evolutionary Microbiology
.2026;[Epub] CrossRef - Aquimarina rhodophyticola sp. nov. and Aquimarina besae sp. nov., Isolated from Marine Red Algae
Jeong Min Kim, Byeong Jun Choi, Hülya Bayburt, Dong Min Han, Che Ok Jeon
Current Microbiology.2025;[Epub] CrossRef - Carotenoid-Producing Qipengyuania algicola sp. nov. and Qipengyuania rhodophyticola sp. nov., Isolated from Marine Algae, and Emended Description of the Genus Qipengyuania Xu et al. 2020
Jae Kyeong Lee, Min Woo Lee, Chae Yeong Moon, Jeong Min Kim, Hülya Bayburt, Byeong Jun Choi, Che Ok Jeon
Journal of Microbiology and Biotechnology.2025;[Epub] CrossRef -
Flagellimonas ulvae sp. nov. and Flagellimonas rhodophyticola sp. nov., isolated from marine algae
Hui Seong Won, Dong Min Han, Jeong Min Kim, Hülya Bayburt, Byeong Jun Choi, Zhe-Xue Quan, Che Ok Jeon
International Journal of Systematic and Evolutionary Microbiology
.2025;[Epub] CrossRef - Validation List no. 226: valid publication of new names and new combinations effectively published outside the IJSEM
Aharon Oren, Markus Göker
International Journal of Systematic and Evolutionary Microbiology
.2025;[Epub] CrossRef






 ePub Link
ePub Link Cite this Article
Cite this Article
 MSK
MSK








