Articles
- Page Path
- HOME > J. Microbiol > Volume 63(10); 2025 > Article
-
Full article
Pycnogenol reduces the expression of P. aeruginosa T3SS and inflammatory response in NCI-H292 cells - Seung-Ho Kim1,2, Da Yun Seo5, Sang-Bae Han3, Un-Hwan Ha6, Ji-Won Park4,7,*, Kyung-Seop Ahn1,*
-
Journal of Microbiology 2025;63(10):e2503004.
DOI: https://doi.org/10.71150/jm.2503004
Published online: September 19, 2025
1Natural Medicine Research Center, Korea Research Institute of Bioscience and Biotechnology, Chungbuk 28116, Republic of Korea
2College of Life Sciences and Biotechnology, Korea University, Seoul 02841, Republic of Korea
3College of Pharmacy, Chungbuk National University, Chungbuk 28160, Republic of Korea
4Division of Practical Research, Honam National Institute of Biologucal Resources (HNIBR), Jeollanam-do 58762, Republic of Korea
5ELEO Inc, Cheongju-si 28160, Republic of Korea
6Department of Biotechnology and Bioinformatics, Korea university, Sejong 30019, Republic of Korea
7Advanced Research Center for Island Wildlife Biomaterials, Honam National Institute of Biological Resources (HNIBR), Jeollanam-do 58762, Republic of Korea
-
*Correspondence Ji-Won Park jjiwon87@gmail.com, jjiwon2@hnibr.re.kr
*Kyung-Seop Ahn ksahn@kribb.re.kr
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,214 Views
- 38 Download
ABSTRACT
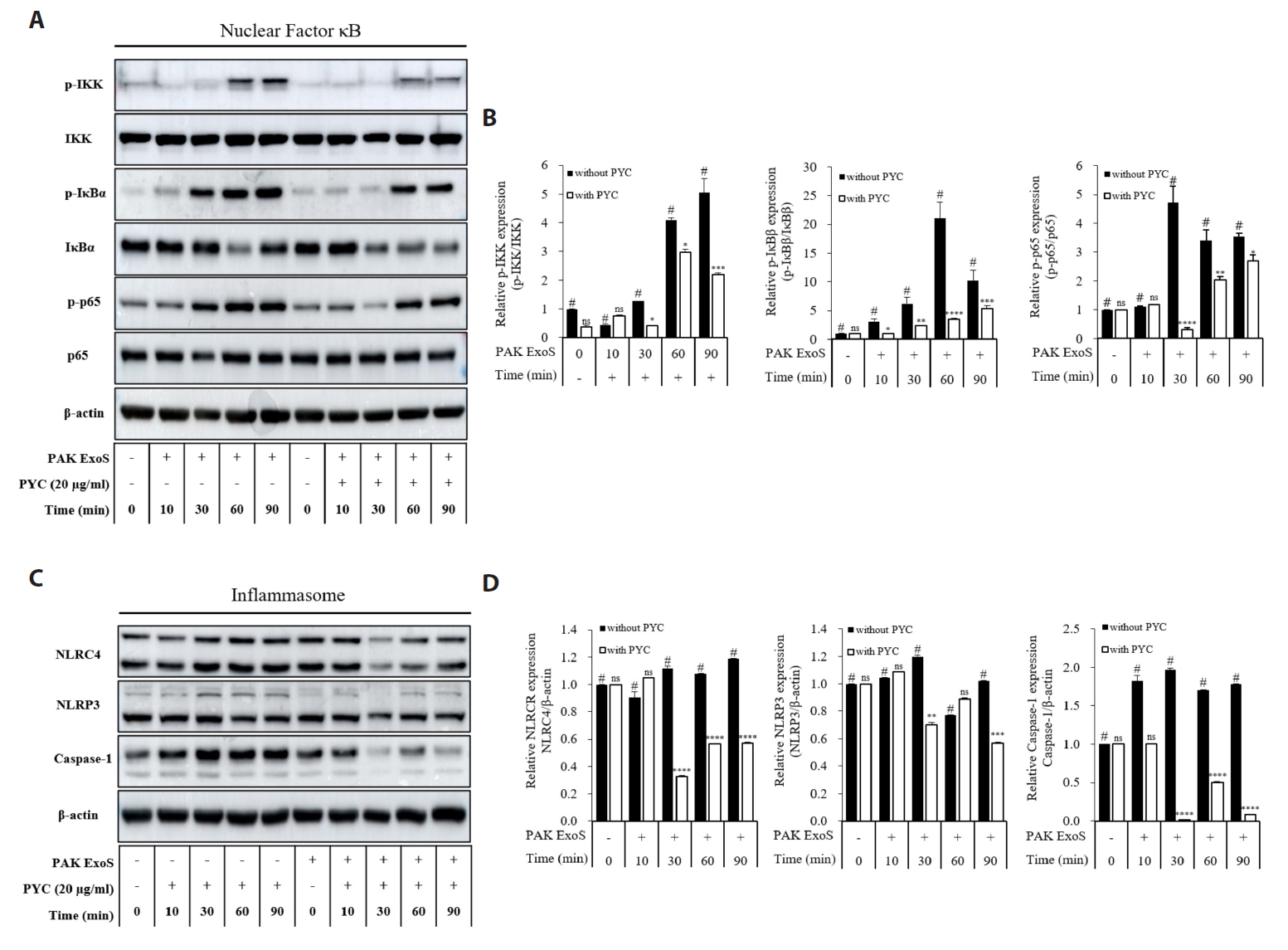
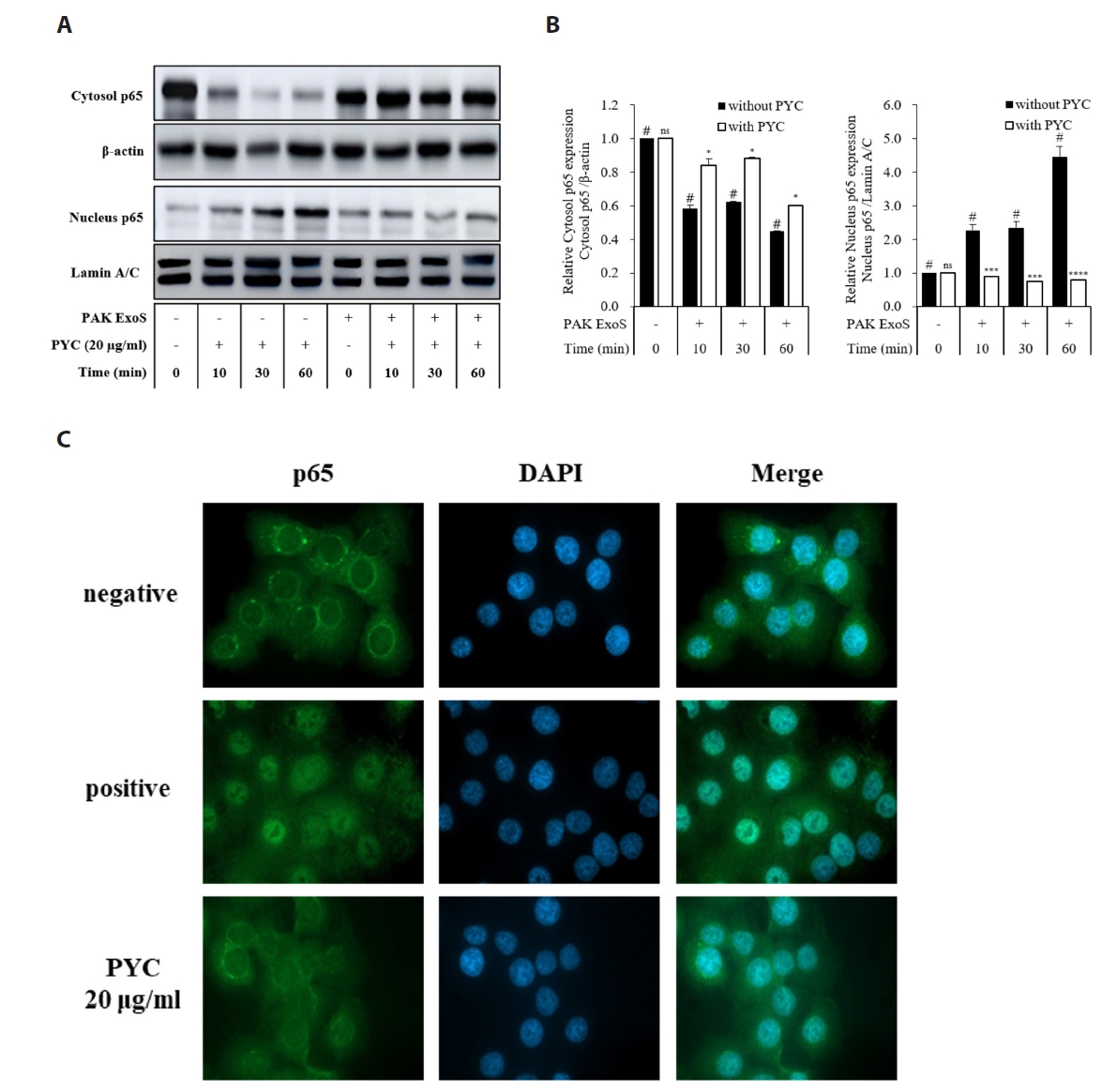
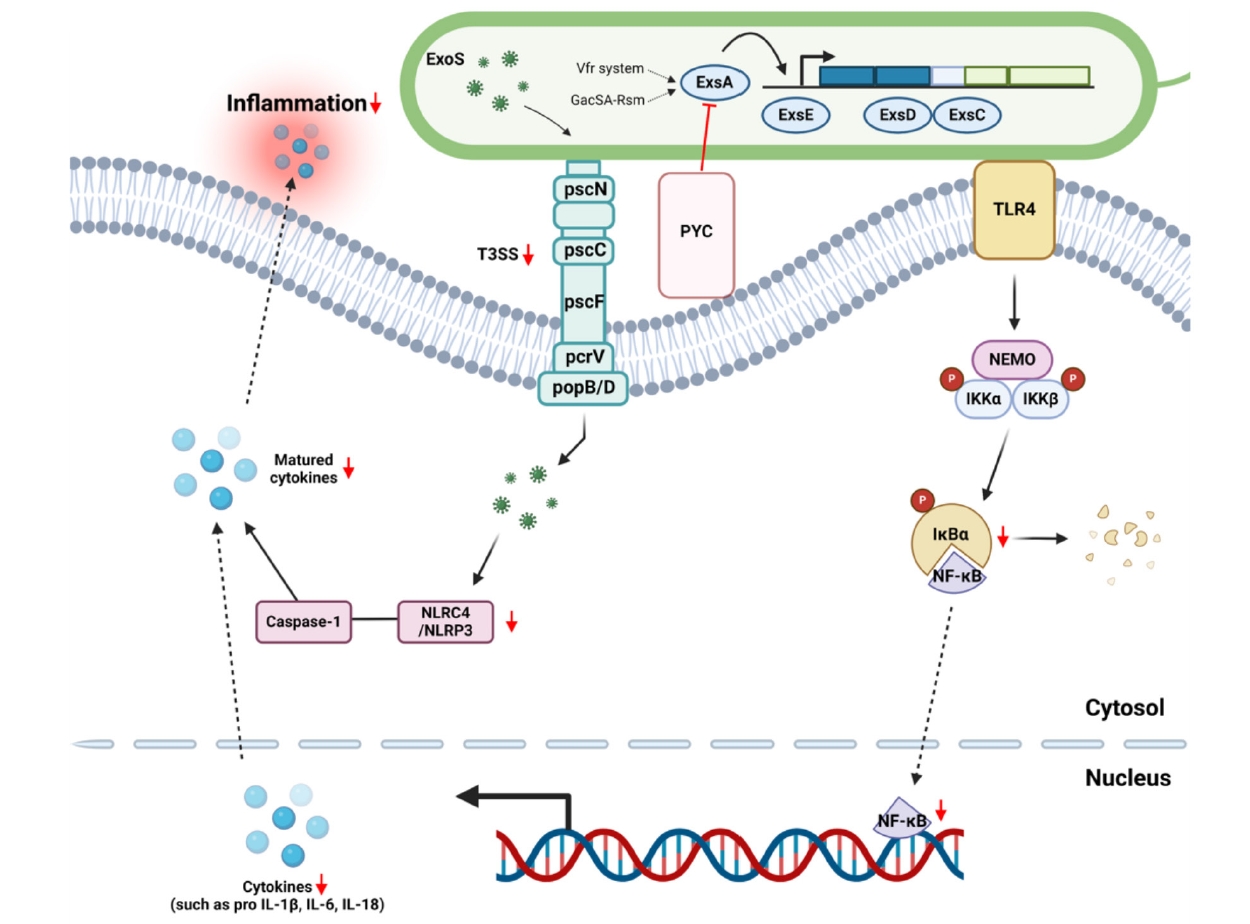
- Nosocomial infections caused by Pseudomonas aeruginosa (P. aeruginosa) have become increasingly common, particularly among immunocompromised individuals, who experience high mortality rates and prolonged treatment durations due to the limited availability of effective therapies. In this study, we screened for anti-ExoS compounds targeting P. aeruginosa and identified pycnogenol (PYC) as a potent inhibitor of the type III secretion system (T3SS), a major virulence mechanism responsible for the translocation of effectors such as ExoS. Using ELISA, western blotting, and real-time PCR analyses in both P. aeruginosa and infected H292 cells, we found that PYC significantly reduced T3SS activity. Mechanistically, PYC suppressed the transcription of T3SS-related genes by downregulating exsA expression in P. aeruginosa. Furthermore, pretreatment with PYC attenuated the cytotoxic effects and reduced the expression of proinflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-18 (IL-18), in P. aeruginosa-infected H292 cells. These effects were associated with the inhibition of NF-κB signaling and inflammasome activation. Taken together, our findings suggest that PYC may serve as a promising therapeutic candidate against P. aeruginosa infections by targeting T3SS-mediated virulence and modulating host inflammatory responses.
Introduction
Materials and Methods
Results
Discussion
Conclusion
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2025-00523419). Also, this study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (2020R1A2C2101228). Additional support was provided by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM1202511).
Author Contributions
SHK and JWP carried out all experiments and prepared the manuscript. DYS assisted with the in vitro experiments. KSA and SBH prepared and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
Supplementary Information
Fig. S1.
Fig. S2.
- Ahn HI, Jang HJ, Kwon OK, Kim JH, Oh JH, et al. 2023. Quercetin attenuates the production of pro-inflammatory cytokines in H292 human lung epithelial cells infected with Pseudomonas aeruginosa by modulating ExoS production. J Microbiol Biotechnol. 33: 430–440. ArticlePubMedPMC
- Armentrout EI, Rietsch A. 2016. The type III secretion translocation pore senses host cell contact. PLoS Pathog. 12: e1005530. ArticlePubMedPMC
- Azimi S, Kafil HS, Baghi HB, Shokrian S, Najaf K, et al. 2016. Presence of exoY, exoS, exoU and exoT genes, antibiotic resistance and biofilm production among Pseudomonas aeruginosa isolates in Northwest Iran. GMS Hyg Infect Control. 11: Doc04.ArticlePubMedPMC
- Bickel M. 1993. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 64: 456–460. PubMed
- Brutinel ED, Vakulskas CA, Yahr TL. 2010. ExsD inhibits expression of the Pseudomonas aeruginosa type III secretion system by disrupting ExsA self-association and DNA binding activity. J Bacteriol. 192: 1479–1486. ArticlePubMedPDF
- Chen L, Zou Y, Kronfl AA, Wu Y. 2020. Type VI secretion system of Pseudomonas aeruginosa is associated with biofilm formation but not environmental adaptation. Microbiologyopen. 9: e991. ArticlePubMedPMCPDF
- Cohen TS, Prince AS. 2013. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 123: 1630–1637. ArticlePubMedPMC
- Czechowska K, McKeithen-Mead S, Al Moussawi K, Kazmierczak BI. 2014. Cheating by type 3 secretion system-negative Pseudomonas aeruginosa during pulmonary infection. Proc Natl Acad Sci USA. 111: 7801–7806. ArticlePubMedPMC
- Filloux A. 2011. Protein secretion systems in Pseudomonas aeruginosa: An essay on diversity, evolution, and function. Front Microbiol. 2: 155.ArticlePubMedPMC
- Frank DW, Nair G, Schweizer HP. 1994. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 62: 554–563. ArticlePubMedPMCPDF
- Galliher-Beckley AJ, Lan LQ, Aono S, Wang L, Shi J. 2013. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J Biol Chem. 4: 30–34. ArticlePubMedPMC
- Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 274: 36369–36372. ArticlePubMed
- Hardy KS, Tessmer MH, Frank DW, Audia JP. 2021. Perspectives on the Pseudomonas aeruginosa type III secretion system effector ExoU and its subversion of the host innate immune response to infection. Toxins (Basel). 13: 880.ArticlePubMedPMC
- Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat Rev Microbiol. 7: 654–665. ArticlePubMedPMCPDF
- Ihim SA, Abubakar SD, Zian Z, Sasaki T, Saffarioun M, et al. 2022. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: biological role in induction, regulation, and treatment. Front Immunol. 13: 919973.ArticlePubMedPMC
- Jansson AL, Yasmin L, Warne P, Downward J, Palmer RH, et al. 2006. Exoenzyme S of Pseudomonas aeruginosa is not able to induce apoptosis when cells express activated proteins, such as Ras or protein kinase B/Akt. Cell Microbiol. 8: 815–822. ArticlePubMed
- Karmakar M, Sun Y, Hise AG, Rietsch A, Pearlman E. 2012. Cutting edge: IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J Immunol. 189: 4231–4235. ArticlePubMedPDF
- Kaufman MR, Jia J, Zeng L, Ha U, Chow M, et al. 2000. Pseudomonas aeruginosa-mediated apoptosis requires the ADP-ribosylating activity of ExoS. Microbiology. 146: 2531–2541. ArticlePubMed
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, et al. 2008. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science. 321: 259–263. ArticlePubMed
- Lee MS, Moon KY, Bae DJ, Park MK, Jang AS. 2013. The effects of pycnogenol on antioxidant enzymes in a mouse model of ozone exposure. Korean J Intern Med. 28: 216–223. ArticlePubMedPMC
- Lin CK, Lee DSW, McKeithen-Mead S, Emonet T, Kazmierczak B. 2021. A primed subpopulation of bacteria enables rapid expression of the type 3 secretion system in Pseudomonas aeruginosa. mBio. 12: e0083121. ArticlePubMedPDF
- Liu T, Zhang L, Joo D, Sun SC. 2017. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2: 17023.ArticlePubMedPMCPDF
- Mancl JM, Suarez C, Liang WG, Kovar DR, Tang WJ. 2020. Pseudomonas aeruginosa exoenzyme Y directly bundles actin filaments. J Biol Chem. 295: 3506–3517. ArticlePubMedPMC
- Morales E, Cots F, Sala M, Comas M, Belvis F, et al. 2012. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv Res. 12: 122.ArticlePubMedPMCPDF
- Mustafi S, Rivero N, Olson JC, Stahl PD, Barbieri MA. 2013. Regulation of Rab5 function during phagocytosis of live Pseudomonas aeruginosa in macrophages. Infect Immun. 81: 2426–2436. ArticlePubMedPMCPDF
- Rocha CL, Coburn J, Rucks EA, Olson JC. 2003. Characterization of Pseudomonas aeruginosa exoenzyme S as a bifunctional enzyme in J774A.1 macrophages. Infect Immun. 71: 5296–5305. ArticlePubMedPMCPDF
- Saleeb M, Sundin C, Aglar Ö, Pinto AF, Ebrahimi M, et al. 2018. Structure-activity relationships for inhibitors of Pseudomonas aeruginosa exoenzyme S ADP-ribosyltransferase activity. Eur J Med Chem. 143: 568–576. ArticlePubMed
- Sarges EDSNF, Rodrigues YC, Furlaneto IP, de Melo MVH, Brabo GLDC, et al. 2020. Pseudomonas aeruginosa type III secretion system virulotypes and their association with clinical features of cystic fibrosis patients. Infect Drug Resist. 13: 3771–3781. ArticlePubMedPMCPDF
- Tanaka T, Narazaki M, Kishimoto T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6: a016295.ArticlePubMedPMC
- Torras MAC, Faura CA, Schönlau F, Rohdewald P. 2005. Antimicrobial activity of Pycnogenol®. Phytother Res. 19: 647–648. ArticlePubMed
- Ventola CL. 2015. The antibiotic resistance crisis: part 1: causes and threats. PT. 40: 277–283. PubMedPMC
- Wang Y, Yu C, Pan Y, Li J, Zhang Y, et al. 2011. A novel compound C12 inhibits inflammatory cytokine production and protects from inflammatory injury in vivo. PLoS One. 6: e24377. ArticlePubMedPMC
- Wilson D, Evans M, Guthrie N, Sharma P, Baisley J, et al. 2010. A randomized, double-blind, placebo-controlled exploratory study to evaluate the potential of Pycnogenol® for improving allergic rhinitis symptoms. Phytother Res. 24: 1115–1119. ArticlePubMed
- Yahr TL, Mende-Mueller LM, Friese MB, Frank DW. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 179: 7165–7168. ArticlePubMedPMCPDF
- Yang JJ, Tsuei KC, Shen EP. 2022. The role of type III secretion system in the pathogenesis of Pseudomonas aeruginosa microbial keratitis. Tzu Chi Med J. 34: 8–14. ArticlePubMed
- Zhao YH, Shaw JG. 2016. Cross-talk between the Aeromonas hydrophila type III secretion system and lateral flagella system. Front Microbiol. 7: 1434.ArticlePubMedPMC
- Zhu M, Zhao J, Kang H, Kong W, Liang H. 2016. Modulation of type III secretion system in Pseudomonas aeruginosa: involvement of the PA4857 gene product. Front Microbiol. 7: 7.ArticlePubMedPMC
References
Figure & Data
References
Citations

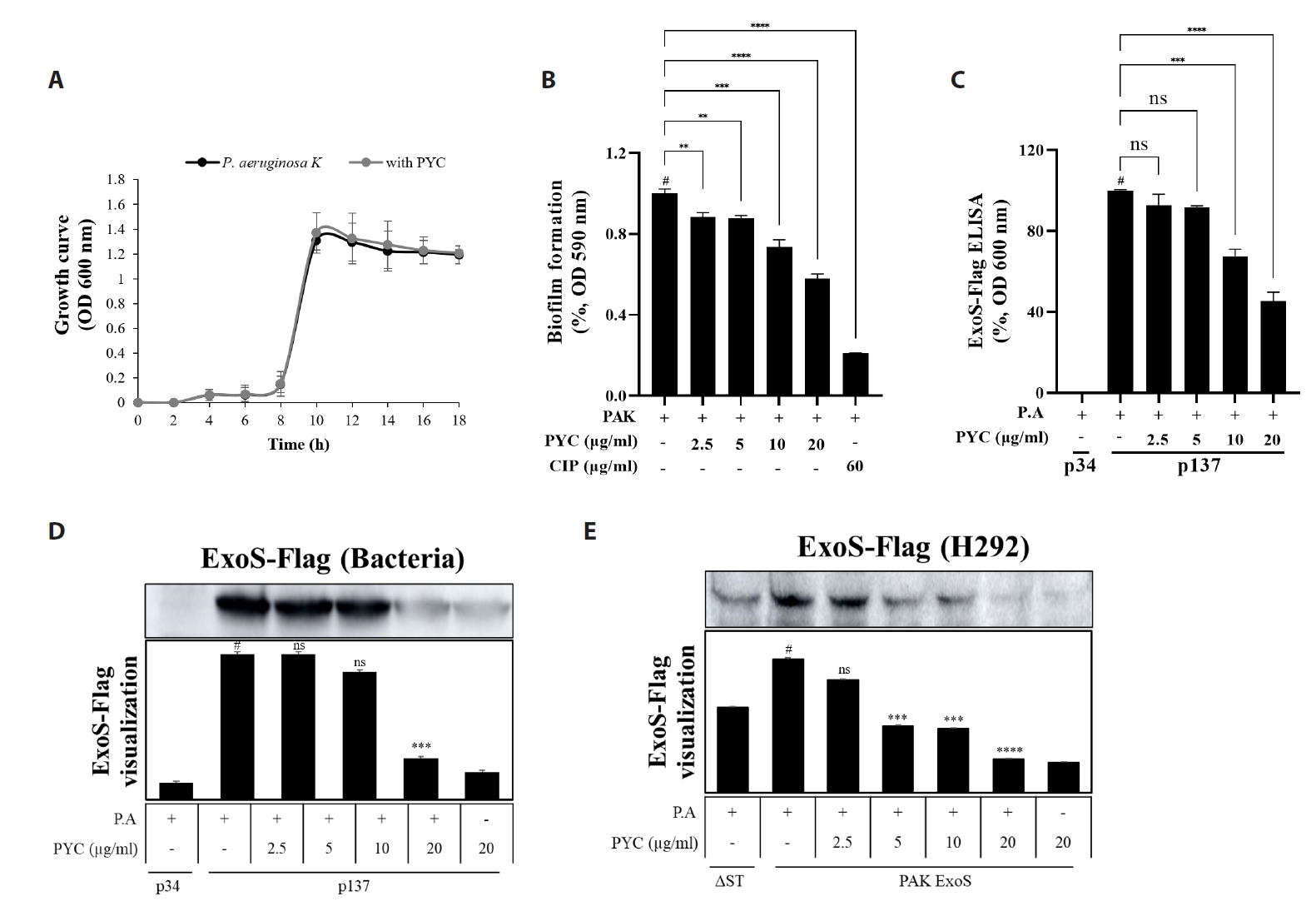
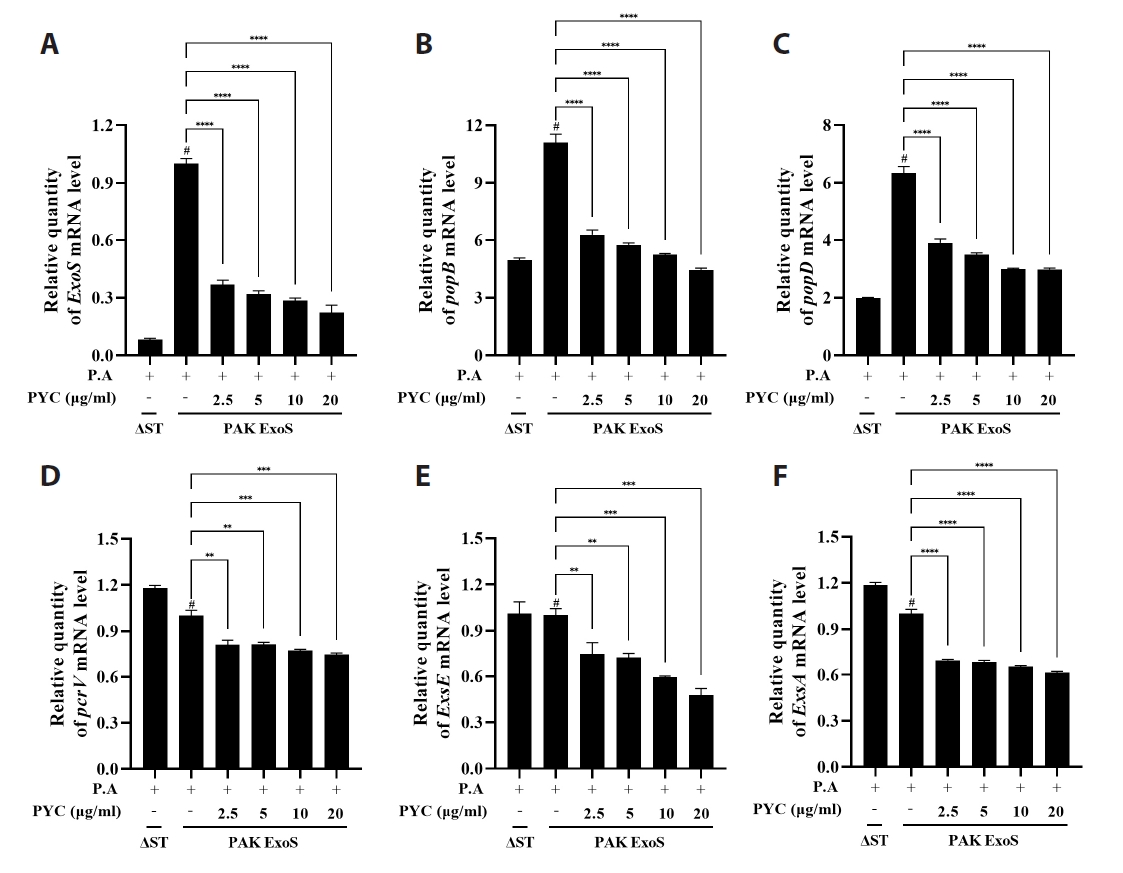
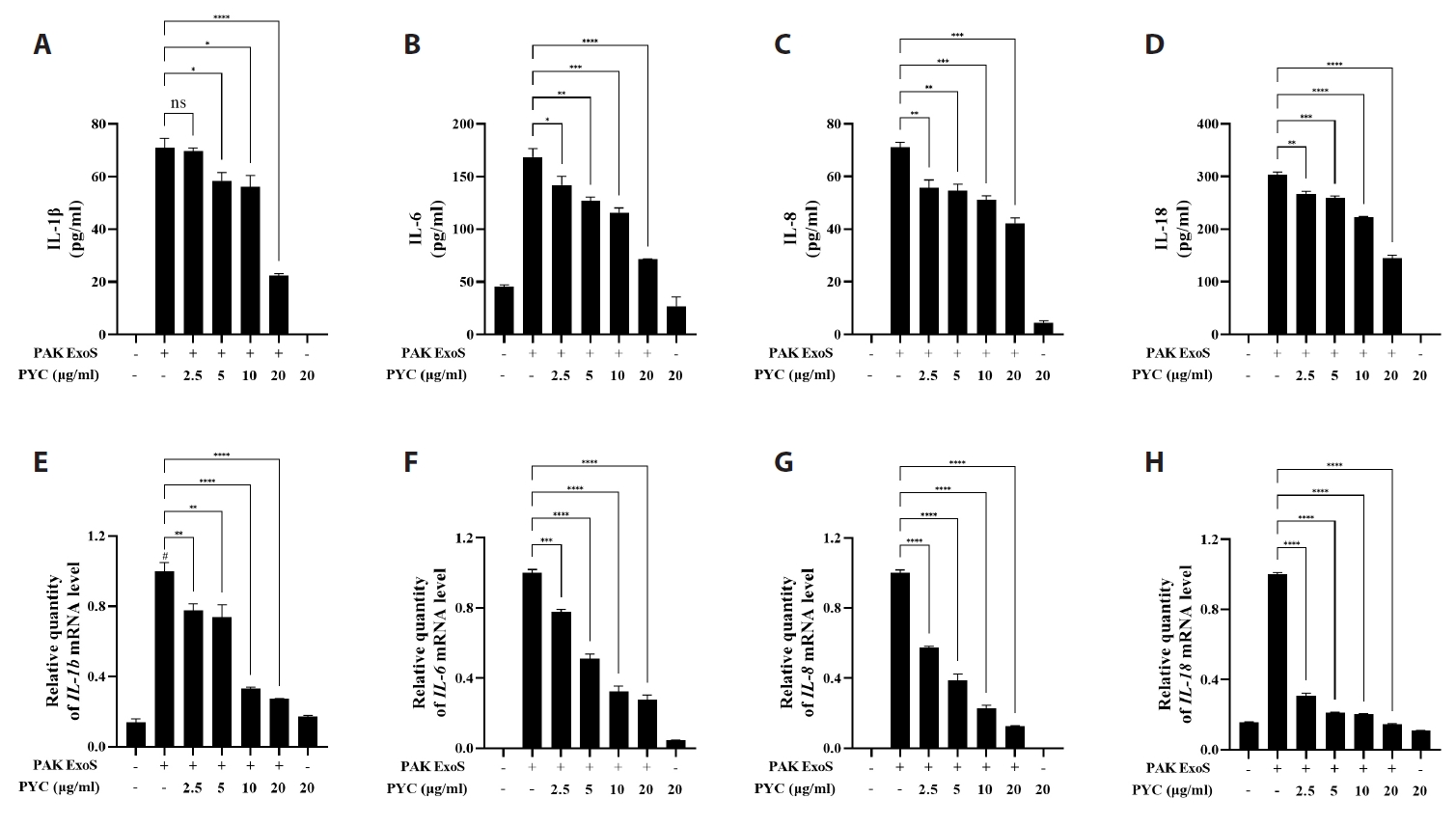
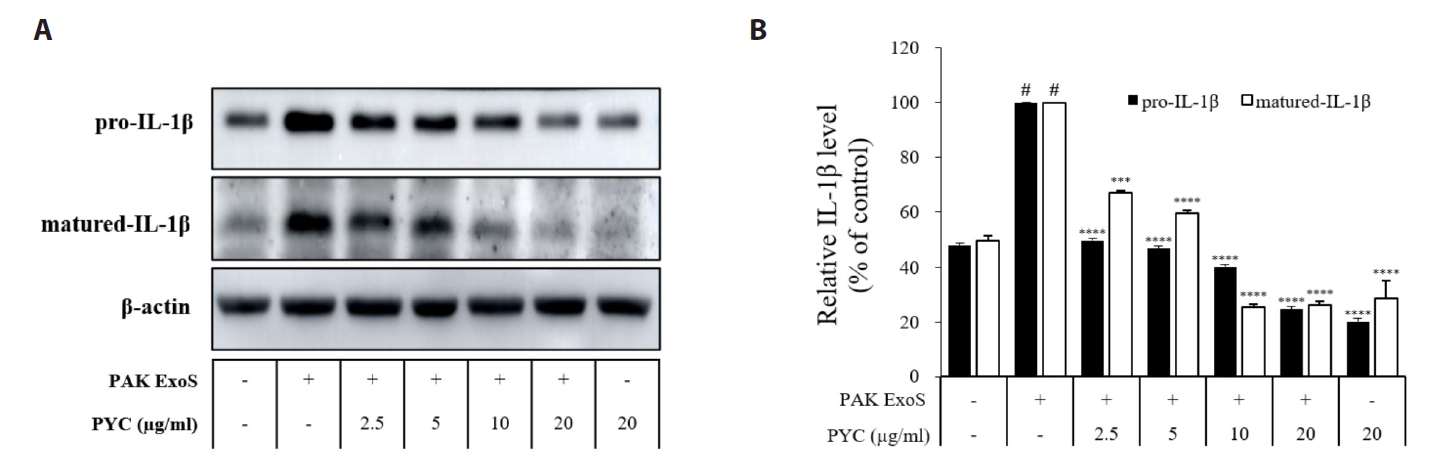
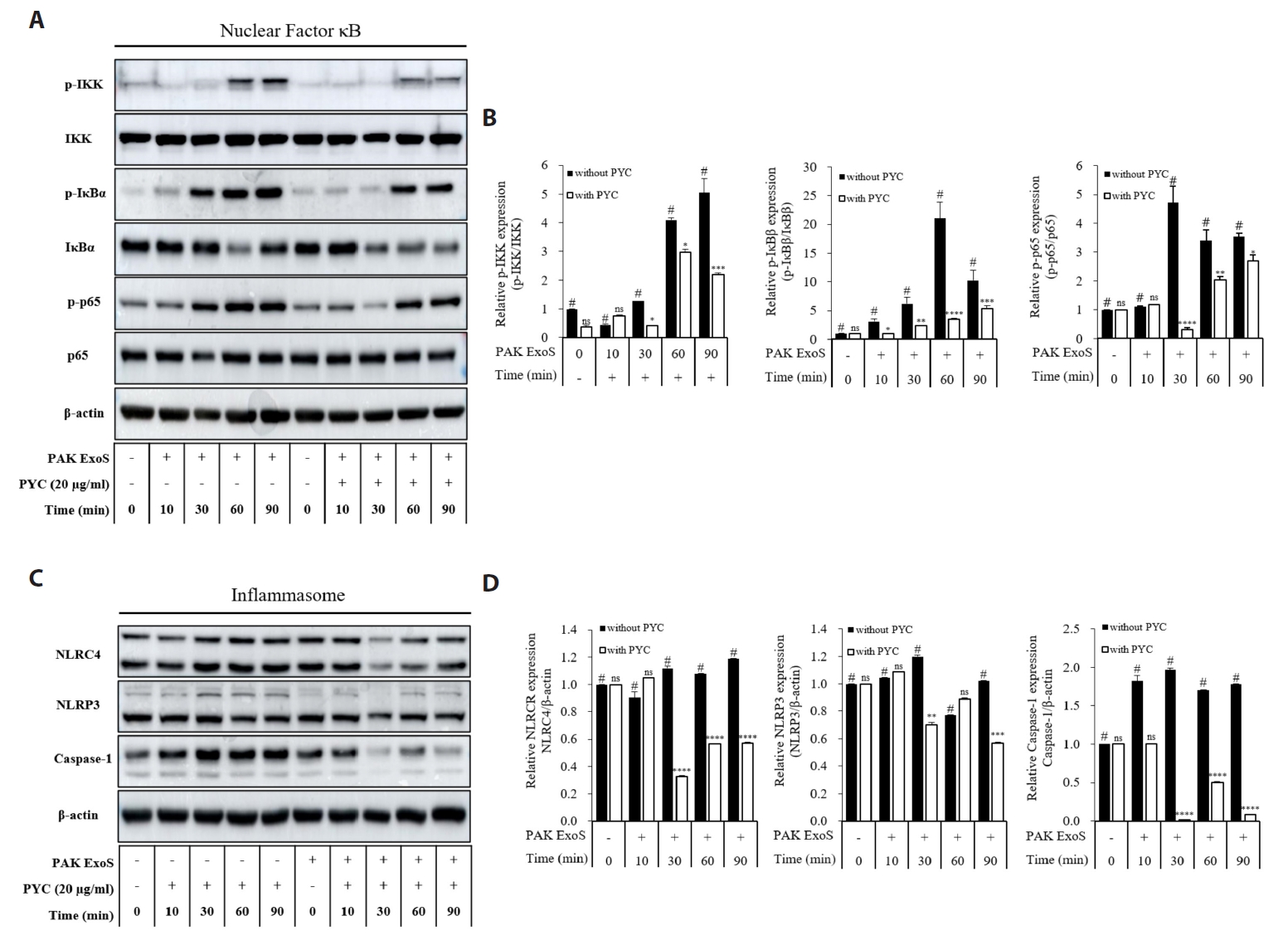
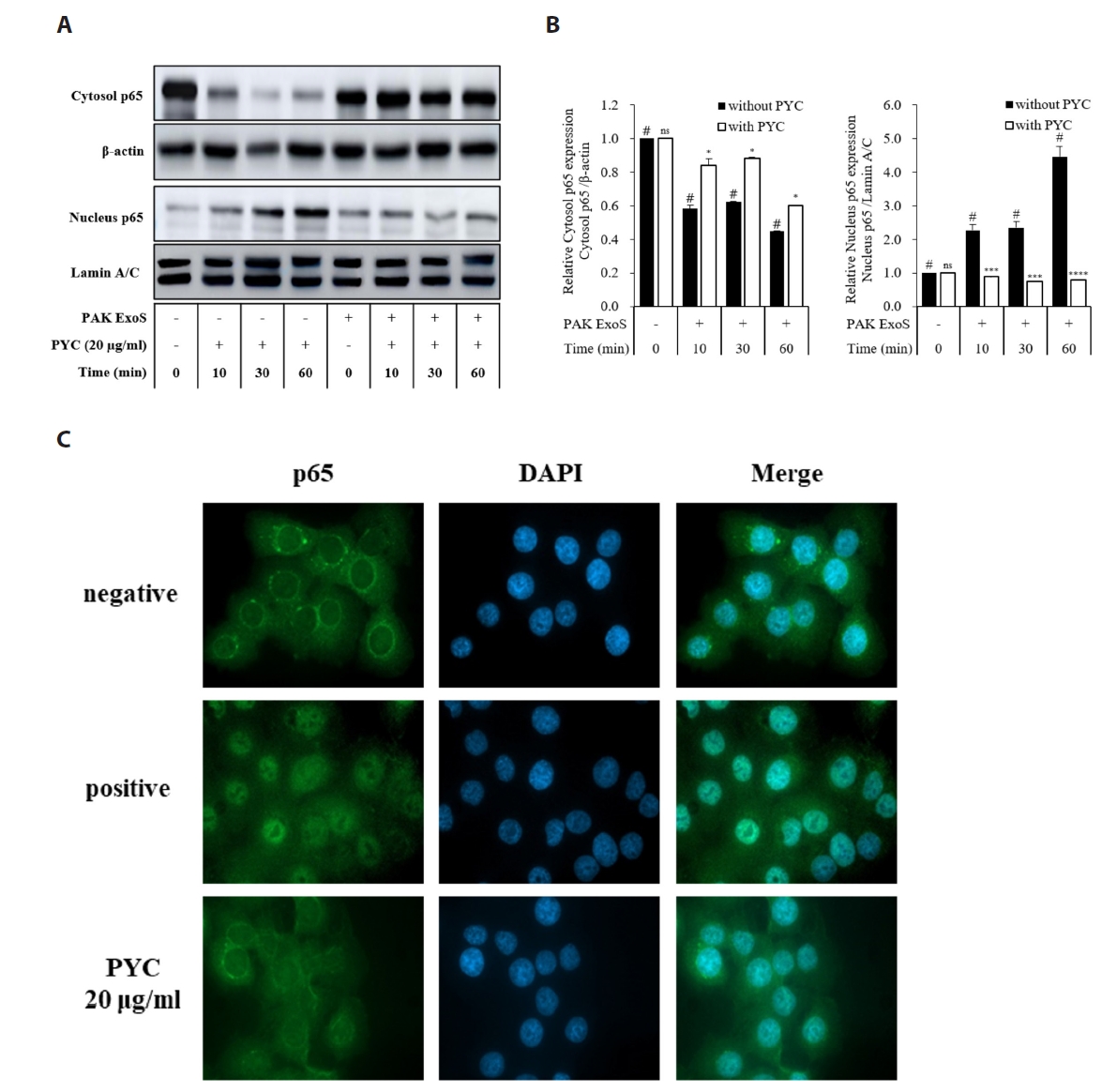
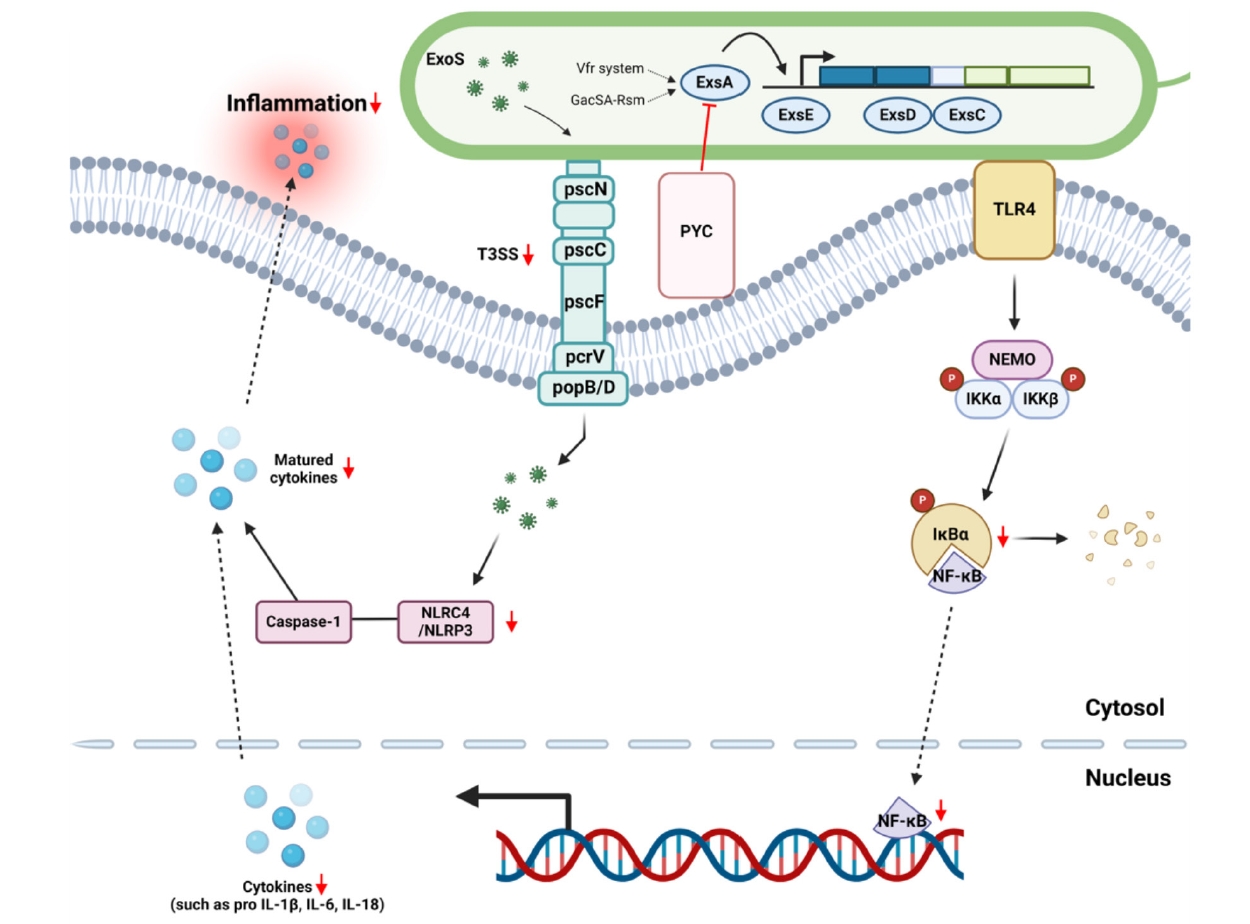
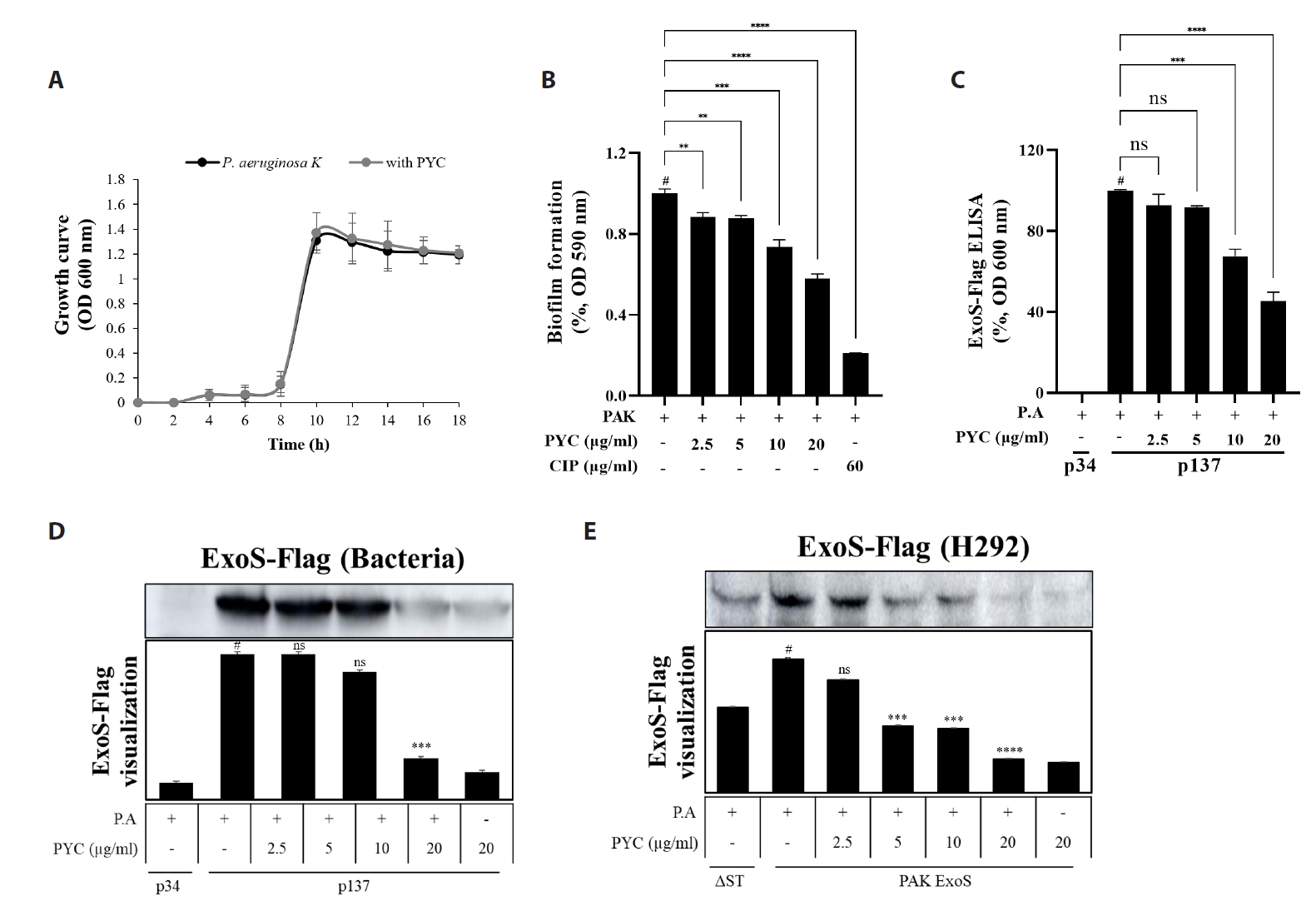
Fig. 1.
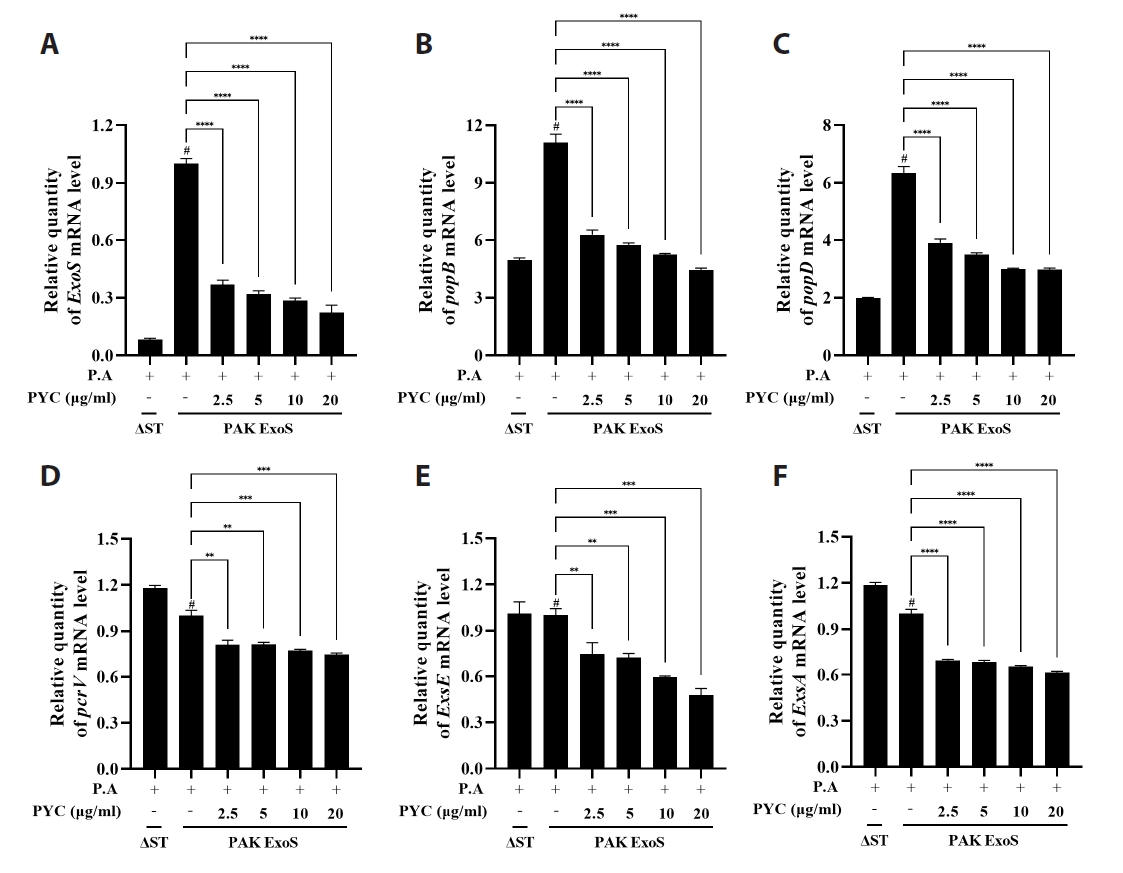
Fig. 2.
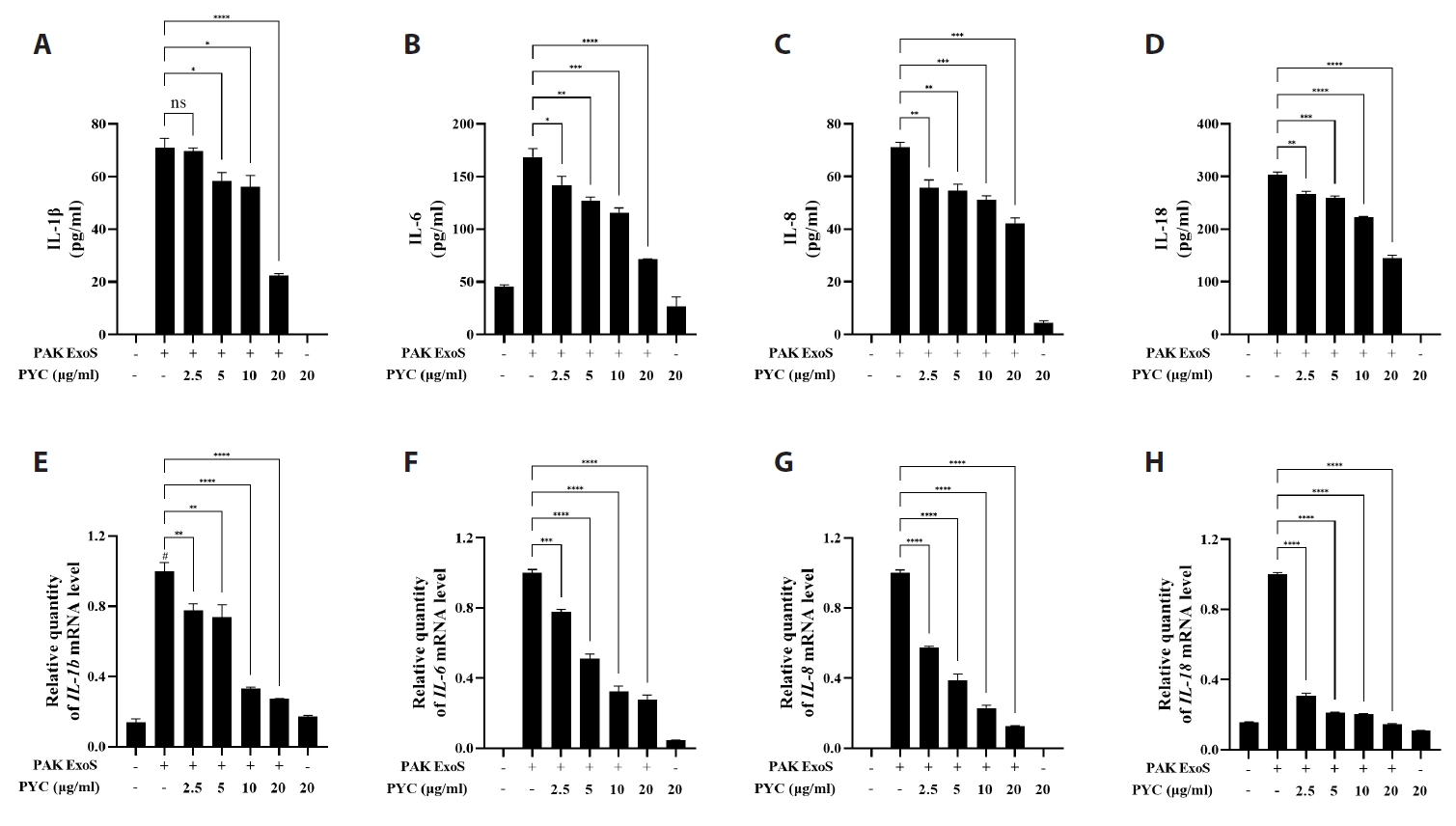
Fig. 3.
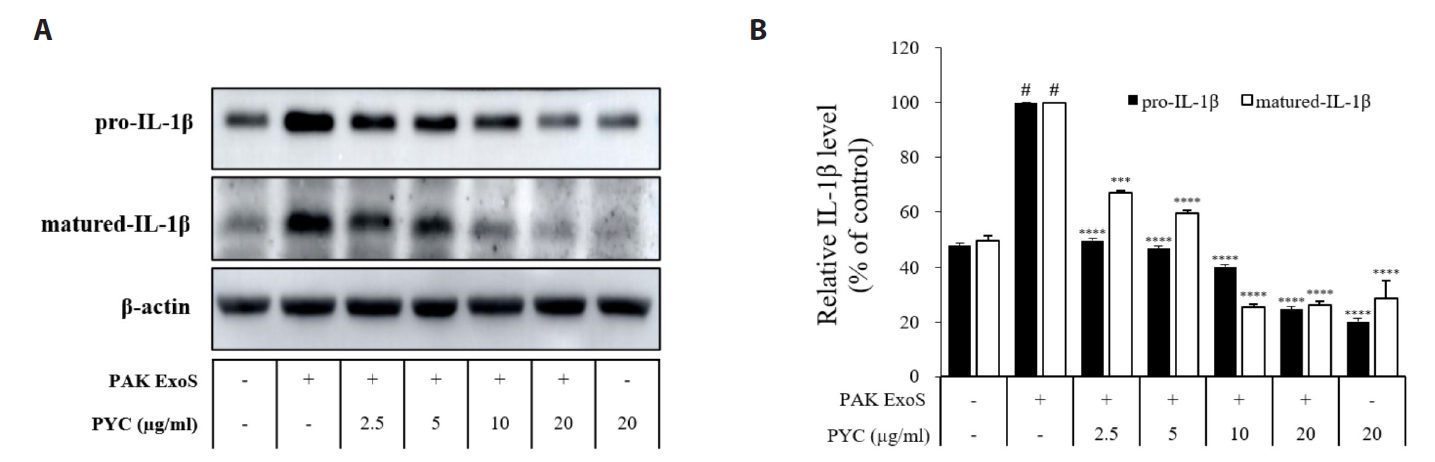
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
| Strains | Description | Antibiotics | Source |
|---|---|---|---|
| P. aeruginosa K (PAK) | Clinical isolate of Wild-type invasive strain | - | From Dr. Ha (Korea University) |
| PAK exsA::Ω/pHW0029 (p34; negative control) | PAK with chromosonal disruption of the exsA loci with Ω cassette | Sp200, Sm200, Gm200, Cb150 | From Dr. Ha |
| PAK exoST::Ω/pHW0225 (p137) | PAK ExoST double mutated exoS-FLAG | Sp200, Gm200, Cb150 | From Dr. Ha |
| PAK ΔST | Double effector mutant; functional needle and translocon apparatus without known effectors | - | From Dr. Ha |
| PAK ΔST/pUCP18 (PAK ExoS) | PAK ΔST with exoS of pUCP18 | Cb150 | From Dr. Park |
| P. aeruginosa primers | Sense (5’→3’) | Anti-sense (5’→3’) |
|---|---|---|
| 16S | CAA AAC TAC TGA GCT AGA GTA CG | GCC ACT GGT GTT CCT TCC TA |
| ExoS | CAG GCT GAA CAG GTA GTG AA | TTC AGG GAG GTG GAG AGA TA |
| popB | GCG CTT CGA CGC TGT TGT | TTC TTC CGA CTC CCT GAT CTT CT |
| popD | GAA GAC CCT GCA GAA GAA CA | ACC TTG CCG ACG ATC TTG |
| pcrV | GAT CGA CGC TGG CGG TAT | TCA TCG CTG AGG CCC TTG |
| ExsE | TGC TGT TCG ACG AAC AGG TG | ATC GTT TGC ATC GCT CCC TG |
| ExsA | AAG GAG CCA AAT CTC TTG | CTT GTT TAC CCT GTA TTC G |
| Human primers | ||
| GAPDH | GCA GGG GGG AGC CAA AAG GG | TGC CAG CCC CAG CGT CAA AG |
| IL-1β | GG ACA AGC TGA GGA AGA TGC | TC TTT CAA CAC GCA GGA CAG |
| IL-6 | GAC AGC CAC TCA CCT CTT CA | AGT GCC TCT TTG CTG CTT TC |
| IL-8 | ATG ACT TCC AAG CTG GTG GCT | TTA TGA ATT CTC AGC CCT CTT CAA AAA |
| IL-18 | GCT TTG GCC TTG GAA GAT GA | GAA GAT TCA AAT TGC ATC TTA T |
Summary of
Table 1.
Table 2.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article








